

Background: Guselkumab (GUS), a monoclonal antibody that specifically binds to the p19-subunit of IL-23, is approved to treat psoriasis. Through Week24 (W24) of the Ph3, double-blind, placebo (PBO)-controlled trial in biologic-naïve pts with active PsA (DISCOVER-2), GUS every 4 or 8 weeks (Q4W or Q8W) demonstrated efficacy for joint & skin symptoms and inhibition of structural damage progression (Q4W), and was well tolerated.
Objectives: Assess GUS efficacy and safety through W52.
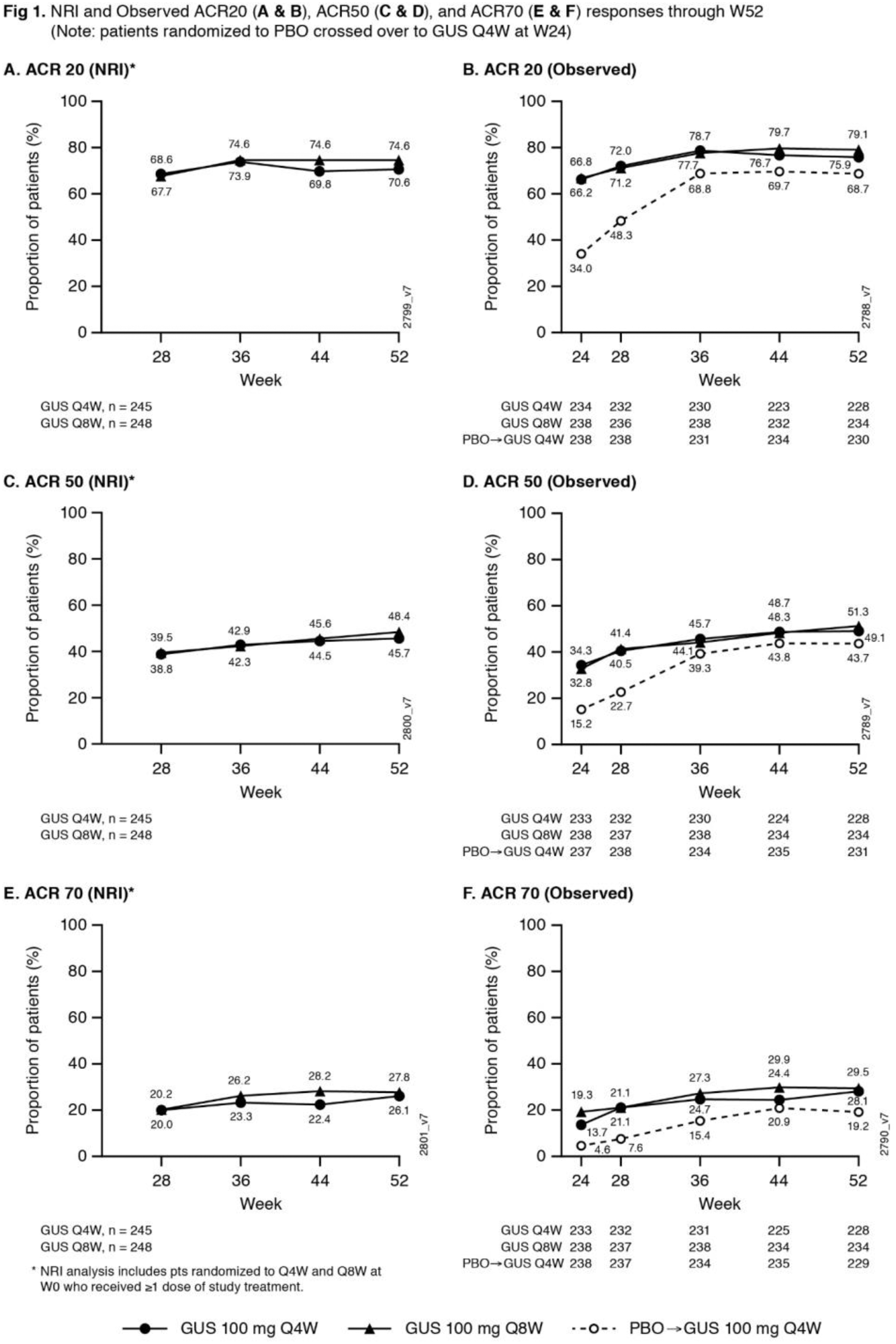
Methods: Biologic-naïve adults with active PsA (≥5 swollen+≥5 tender joints; CRP ≥0.6mg/dL) were randomized (1:1:1) to GUS 100 mg Q4W; GUS 100 mg at W0, W4, Q8W; or PBO. At W24, PBO pts crossed over to GUS 100 mg Q4W (PBO X Q4W). ACR response rates at W52, based on nonresponder imputation (NRI) for missing data and as observed in pts who continued study agent at W24, are shown. Observed data for additional endpoints, including PsA-modified van der Heijde Sharp (vdH-S) scores derived from blinded radiographic images collected at W0, W24, W52 (or at d/c) and scored in a new Read Campaign, are shown.
Results: 712/739 (96.3%) randomized & treated pts continued study agent at W24; 689/739 (93.2%) completed Wk52. NRI ACR20 response rates continued to increase after W24, and at W52 were 70.6% for GUS Q4W and 74.6% for GUS Q8W (
Observed Efficacy 1
| GUS | Q4W | GUS | Q8W |
PBO X
|
GUS Q4W
|
|
|---|---|---|---|---|---|---|
| Data are % unless otherwise stated | W24 | W52 | W24 | W52 | W24 | W52 |
| Dactylitis at W0, n | 116 | 111 | 107 | 105 | 95 | 93 |
| Resolution | 68.1 | 81.1 | 60.7 | 81.9 | 41.1 | 78.5 |
| Enthesitis at W0, n | 165 | 160 | 151 | 148 | 172 | 168 |
| Resolution | 45.5 | 60.0 | 57.6 | 65.5 | 32.6 | 67.3 |
| ≥3% BSA psoriasis, IGA ≥2 at W0, n | 176 | 173 | 172 | 170 | 176 | 172 |
| IGA 0/1 + ≥2-grade decrease | 71.0 | 84.4 | 72.1 | 77.1 | 19.9 | 84.3 |
| PASI75 | 81.8 | 91.9 | 80.8 | 88.8 | 23.3 | 88.4 |
| PASI90 | 63.6 | 81.5 | 70.3 | 77.1 | 10.2 | 76.7 |
| PASI100 | 46.6 | 61.3 | 46.5 | 54.7 | 2.8 | 55.2 |
| HAQ-DI, n | 234 | 229 | 238 | 234 | 237 | 230 |
| Mean change | -0.4 | -0.5 | -0.4 | -0.5 | -0.2 | -0.4 |
| SF-36 scores, n (mean change) | 234 | 229 | 238 | 234 | 237 | 230 |
| Physical Component - PCS | 7.2 | 9.0 | 7.8 | 9.5 | 3.8 | 8.1 |
| Mental Component - MCS | 4.1 | 4.1 | 4.5 | 4.5 | 2.2 | 4.3 |
| MDA/VLDA , n | 234 | 228 | 238 | 234 | 238 | 231 |
| MDA | 19.7 | 36.8 | 26.5 | 32.9 | 6.3 | 31.6 |
| VLDA | 5.1 | 12.2 2 | 4.6 3 | 17.1 | 1.3 | 6.9 |
1 Randomized pts still on study agent at W24; 2 N=229; 3 N=237
Conclusion: In biologic-naïve pts with active PsA, GUS elicited sustained improvements in joint & skin symptoms; inhibition of radiographic progression & improvements in physical function, quality of life & composite indices through W52. GUS safety in PsA was similar at W24 1 & W52 and consistent with GUS safety in psoriasis.
REFERENCES:
[1]Mease P (A#L13), Arthritis Rheumatol 2019;71(suppl 10)
Acknowledgments: None
Disclosure of Interests: Iain McInnes Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, and UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, and UCB, Proton Rahman Grant/research support from: Janssen and Novartis, Consultant of: Abbott, AbbVie, Amgen, BMS, Celgene, Lilly, Janssen, Novartis, and Pfizer., Speakers bureau: Abbott, AbbVie, Amgen, BMS, Celgene, Lilly, Janssen, Novartis, Pfizer, Alice B Gottlieb Grant/research support from:: Research grants, consultation fees, or speaker honoraria for lectures from: Pfizer, AbbVie, BMS, Lilly, MSD, Novartis, Roche, Sanofi, Sandoz, Nordic, Celltrion and UCB., Consultant of:: Research grants, consultation fees, or speaker honoraria for lectures from: Pfizer, AbbVie, BMS, Lilly, MSD, Novartis, Roche, Sanofi, Sandoz, Nordic, Celltrion and UCB., Speakers bureau:: Research grants, consultation fees, or speaker honoraria for lectures from: Pfizer, AbbVie, BMS, Lilly, MSD, Novartis, Roche, Sanofi, Sandoz, Nordic, Celltrion and UCB., Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Ramanand A Subramanian Employee of: Janssen Research & Development, LLC, Prasheen Agarwal Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Shihong Sheng Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Yusang Jiang: None declared, Bei Zhou Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Désirée van der Heijde Consultant of: AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead Sciences, Inc., Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, UCB Pharma; Director of Imaging Rheumatology BV, Philip J Mease Grant/research support from: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – grant/research support, Consultant of: Abbott, Amgen, Biogen Idec, BMS, Celgene Corporation, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical, UCB – consultant, Speakers bureau: Abbott, Amgen, Biogen Idec, BMS, Eli Lilly, Genentech, Janssen, Pfizer, UCB – speakers bureau