

Background: There is a lack of real-life evidence on secukinumab effectiveness in psoriatic arthritis (PsA) patients.
Objectives: To assess the real-life 6- and 12-month secukinumab retention rates and proportions of patients in remission/low disease activity (LDA) overall, and by prior biologic disease-modifying anti-rheumatic drug (bDMARD)/targeted synthetic (ts)DMARD use.
Methods: Data from PsA patients treated with secukinumab in routine care from 13 countries in the European Spondyloarthritis (EuroSpA) Research Collaboration Network were pooled. Patients started secukinumab ≥12 months before date of datacut. Crude and LUNDEX adjusted (crude value adjusted for drug retention) 28-joint Disease Activity index for PSoriatic Arthritis (DAPSA28) and 28-joint Disease Activity Score with CRP (DAS28CRP) remission and LDA rates were calculated. Group comparisons between b/tsDMARD naïve, 1 prior and ≥2 prior b/tsDMARD users were done with ANOVA, Kruskal-Wallis, Chi-square or Kaplan-Meier analyses with log-rank test, as appropriate.
Results: A total of 1543 PsA patients were included (
| All patients (n=1543 ) | b/tsDMARD naïve (n=287 ) | 1 prior b/tsDMARD (n=333 ) | ≥2 prior b/tsDMARDs (n=923 ) | p * | |
|---|---|---|---|---|---|
| Age (years), mean (SD ) | 52 (11) | 49 (12.3) | 51 (11) | 53 (11) | <0.001 |
| Male, % | 42% | 49% | 46% | 39% | 0.003 |
| Years since diagnosis, mean (SD ) | 9 (8) | 7 (8) | 8 (7) | 10 (8) | <0.001 |
| Current smokers, % | 19% | 21% | 22% | 18% | 0.23 |
| CRP (mg/L), median (IQR ) | 5 (2-12) | 7 (2-19) | 4 (2-8) | 5 (2-11) | <0.001 |
| DAPSA28, median (IQR ) | 26 (18-37) | 28 (19-38) | 22 (13-32) | 27 (19-38) | <0.001 |
| DAS28CRP, median (IQR ) | 4.2 (3.3-5.0) | 4.4 (3.5-5.2) | 3.8 (2.6-4.5) | 4.2 (3.4-5.0) | <0.001 |
*Comparisons across number of prior b/tsDMARD were done with ANOVA, Kruskal-Wallis or Chi-square test, as appropriate
| Months | All patients (n=1543) | b/tsDMARD naïve (n=287) | 1 prior b/tsDMARD (n=333) | ≥2 prior b/tsDMARDs (n=923) | p * | |
|---|---|---|---|---|---|---|
| Secukinumab retention rate, % (95%CI) | 6 | 86% (84-87%) | 89% (86-93%) | 85% (81-89%) | 85% (82-87%) | 0.11 |
| 12 | 74% (72-76%) | 81% (76-86%) | 76% (71-80%) | 72% (69-75%) | 0.006 | |
| DAPSA28≤4 | ||||||
| Crude | 6 | 13% | 25% | 11% | 11% | <0.001 |
| LUNDEX | 11% | 22% | 9% | 9% | <0.001 | |
| Crude | 12 | 11% | 22% | 11% | 8% | <0.001 |
| LUNDEX | 7% | 17% | 7% | 5% | 0.001 | |
| DAS28CRP<2.6 | ||||||
| Crude | 6 | 34% | 51% | 33% | 30% | <0.001 |
| LUNDEX | 29% | 45% | 27% | 24% | <0.001 | |
| Crude | 12 | 39% | 55% | 41% | 34% | <0.001 |
| LUNDEX | 26% | 41% | 27% | 21% | <0.001 | |
| DAPSA28 >4 and ≤14 | ||||||
| Crude | 6 | 33% | 42% | 32% | 30% | 0.04 |
| LUNDEX | 27% | 37% | 27% | 25% | 0.02 | |
| Crude | 12 | 35% | 48% | 36% | 32% | 0.009 |
| LUNDEX | 24% | 36% | 24% | 20% | 0.004 | |
| DAS28CRP ≤3.2 | ||||||
| Crude | 6 | 52% | 69% | 53% | 47% | <0.001 |
| LUNDEX | 43% | 61% | 45% | 38% | <0.001 | |
| Crude | 12 | 55% | 72% | 55% | 50% | <0.001 |
| LUNDEX | 37% | 54% | 37% | 32% | <0.001 | |
|
*Comparisons across number of prior b/tsDMARDs were done with Kaplan-Meier with log-rank test or Chi-Square test, as appropriate |
||||||
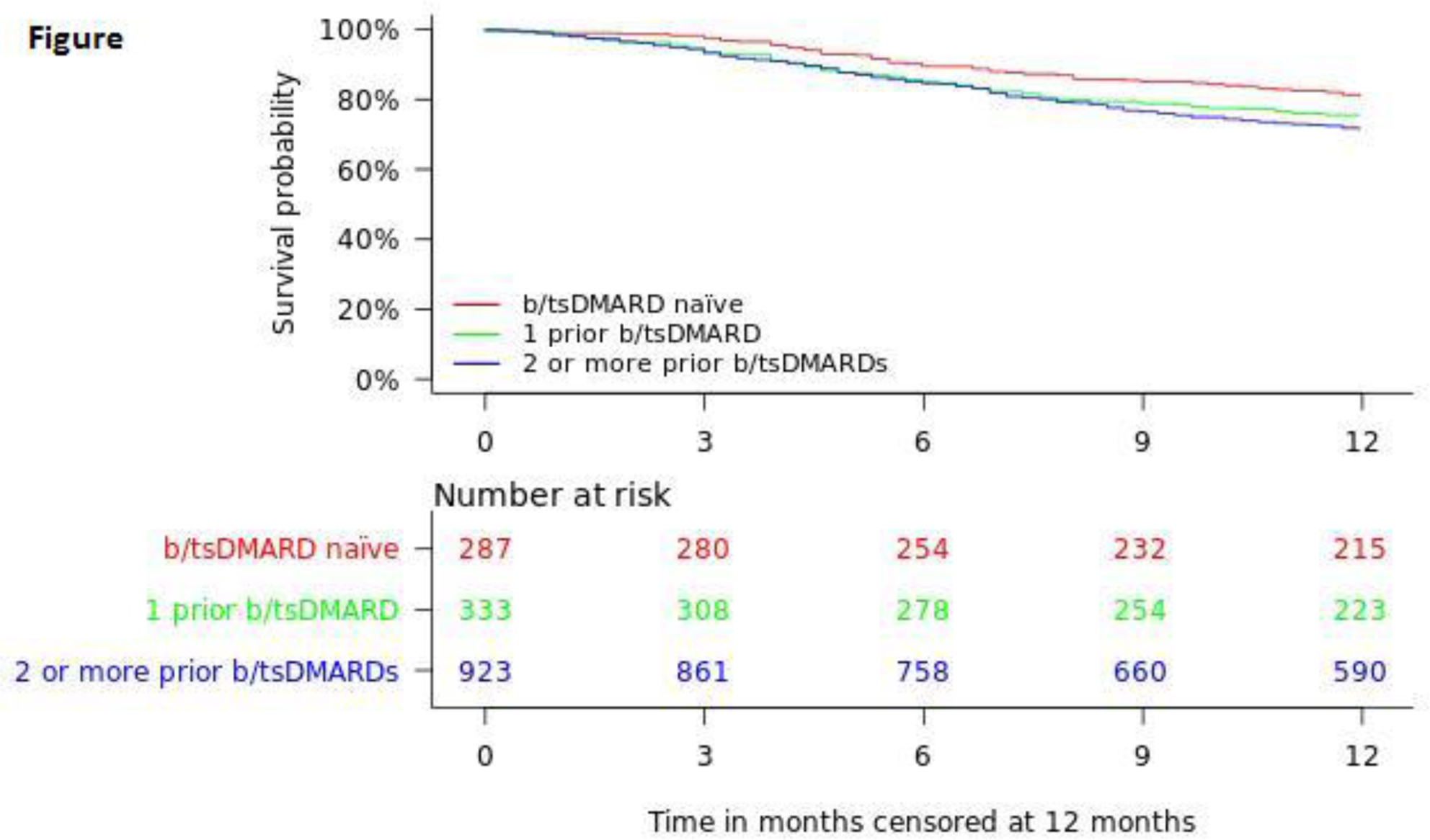
Conclusion: In this real-life study of 1543 patients with PsA in 13 European countries 12-month secukinumab retention was high, and significantly higher for b/tsDMARD naïve patients. Overall, a higher proportion of bionaïve than previous b/tsDMARD users achieved remission, regardless of remission criteria.
Acknowledgments: Novartis and IQVIA for supporting the EuroSpA RCN
Disclosure of Interests: Brigitte Michelsen Grant/research support from: Research support from Novartis, Consultant of: Consulting fees Novartis, Stylianos Georgiadis Grant/research support from: Novartis, Daniela Di Giuseppe: None declared, Anne Gitte Loft Grant/research support from: Novartis, Consultant of: AbbVie, MSD, Novartis, Pfizer and UCB, Speakers bureau: AbbVie, MSD, Novartis, Pfizer and UCB, Michael Nissen Grant/research support from: Abbvie, Consultant of: Novartis, Lilly, Abbvie, Celgene and Pfizer, Speakers bureau: Novartis, Lilly, Abbvie, Celgene and Pfizer, Florenzo Iannone Consultant of: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Speakers bureau: Speaker and consulting fees from AbbVie, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, UCB, MSD, Manuel Pombo-Suarez Consultant of: Janssen, Lilly, MSD and Sanofi., Speakers bureau: Janssen, Lilly, MSD and Sanofi., Heřman Mann: None declared, Ziga Rotar Consultant of: Speaker and consulting fees from Abbvie, Amgen, Biogen, Eli Lilly, Medis, MSD, Novartis, Pfizer, Roche, Sanofi., Speakers bureau: Speaker and consulting fees from Abbvie, Amgen, Biogen, Eli Lilly, Medis, MSD, Novartis, Pfizer, Roche, Sanofi., Kari Eklund Consultant of: Celgene, Lilly, Speakers bureau: Pfizer, Roche, Tore K. Kvien Grant/research support from: Received grants from Abbvie, Hospira/Pfizer, MSD and Roche (not relevant for this abstract)., Consultant of: Have received personal fees from Abbvie, Biogen, BMS, Celltrion, Eli Lily, Hospira/Pfizer, MSD, Novartis, Orion Pharma, Roche, Sandoz, UCB, Sanofi and Mylan (not relevant for this abstract)., Paid instructor for: Have received personal fees from Abbvie, Biogen, BMS, Celltrion, Eli Lily, Hospira/Pfizer, MSD, Novartis, Orion Pharma, Roche, Sandoz, UCB, Sanofi and Mylan (not relevant for this abstract)., Speakers bureau: Have received personal fees from Abbvie, Biogen, BMS, Celltrion, Eli Lily, Hospira/Pfizer, MSD, Novartis, Orion Pharma, Roche, Sandoz, UCB, Sanofi and Mylan (not relevant for this abstract)., Maria Jose Santos Speakers bureau: Novartis and Pfizer, Björn Gudbjornsson Speakers bureau: Novartis and Amgen, Catalin Codreanu Consultant of: Speaker and consulting fees from AbbVie, Accord Healthcare, Alfasigma, Egis, Eli Lilly, Ewopharma, Genesis, Mylan, Novartis, Pfizer, Roche, Sandoz, UCB, Speakers bureau: Speaker and consulting fees from AbbVie, Accord Healthcare, Alfasigma, Egis, Eli Lilly, Ewopharma, Genesis, Mylan, Novartis, Pfizer, Roche, Sandoz, UCB, Sema Yilmaz: None declared, Johan K Wallman Consultant of: AbbVie, Celgene, Eli Lilly, Novartis and UCB Pharma, Cecilie Heegaard Brahe Grant/research support from: Novartis, Burkhard Moeller: None declared, Ennio Giulio Favalli Consultant of: Consultant and/or speaker for BMS, Eli-Lilly, MSD, UCB, Pfizer, Sanofi-Genzyme, Novartis, and Abbvie, Speakers bureau: Consultant and/or speaker for BMS, Eli-Lilly, MSD, UCB, Pfizer, Sanofi-Genzyme, Novartis, and Abbvie, Carlos Sánchez-Piedra: None declared, Lucie Nekvindova: None declared, Matija Tomsic: None declared, Nina Trokovic: None declared, Eirik kristianslund: None declared, Helena Santos Speakers bureau: AbbVie, Eli-Lilly, Janssen, Pfizer, Novartis, Thorvardur Love: None declared, Ruxandra Ionescu Consultant of: Consulting fees from Abbvie, Eli-Lilly, Novartis, Pfizer, Roche, Sandoz, Speakers bureau: Consulting and speaker fees from Abbvie, Eli-Lilly, Novartis, Pfizer, Roche, Sandoz, Yavuz Pehlivan: None declared, Gareth T. Jones Grant/research support from: Pfizer, AbbVie, UCB, Celgene and GSK., Irene van der Horst-Bruinsma Grant/research support from: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Consultant of: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Lykke Midtbøll Ørnbjerg Grant/research support from: Novartis, Mikkel Ǿstergaard Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Merck, and Novartis, Consultant of: AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Hospira, Janssen, Merck, Novartis, Novo Nordisk, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Hospira, Janssen, Merck, Novartis, Novo Nordisk, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB, Merete L. Hetland Grant/research support from: BMS, MSD, AbbVie, Roche, Novartis, Biogen and Pfizer, Consultant of: Eli Lilly, Speakers bureau: Orion Pharma, Biogen, Pfizer, CellTrion, Merck and Samsung Bioepis