

Background: Optic Neuritis (ON) is an inflammation of the optic nerve. Its most common presentation is demyelinating typical ON. Atypical ON is rare, severe, non-demyelinating and can be isolated or associated to different diseases including autoimmune diseases. If it is not treated, it can lead to devastating visual results. Conventional treatment includes systemic corticosteroids and conventional immunosuppressants (CIS).
Objectives: Our aim was to assess the efficacy of biological therapy in atypical ON refractory to conventional treatment.
Methods: Open-label multicenter study including 19 patients diagnosed with atypical ON refractory to systemic corticosteroids and at least one CIS. The main outcomes assessed were Best Corrected Visual Acuity (BCVA) and optic nerve and ganglionar cells Optical Coherence Tomography (OCT). These outcome variables were recorded at baseline, 1 week, 2 weeks, 1 month, 3 months and 6 months and 1 year after biological therapy onset.
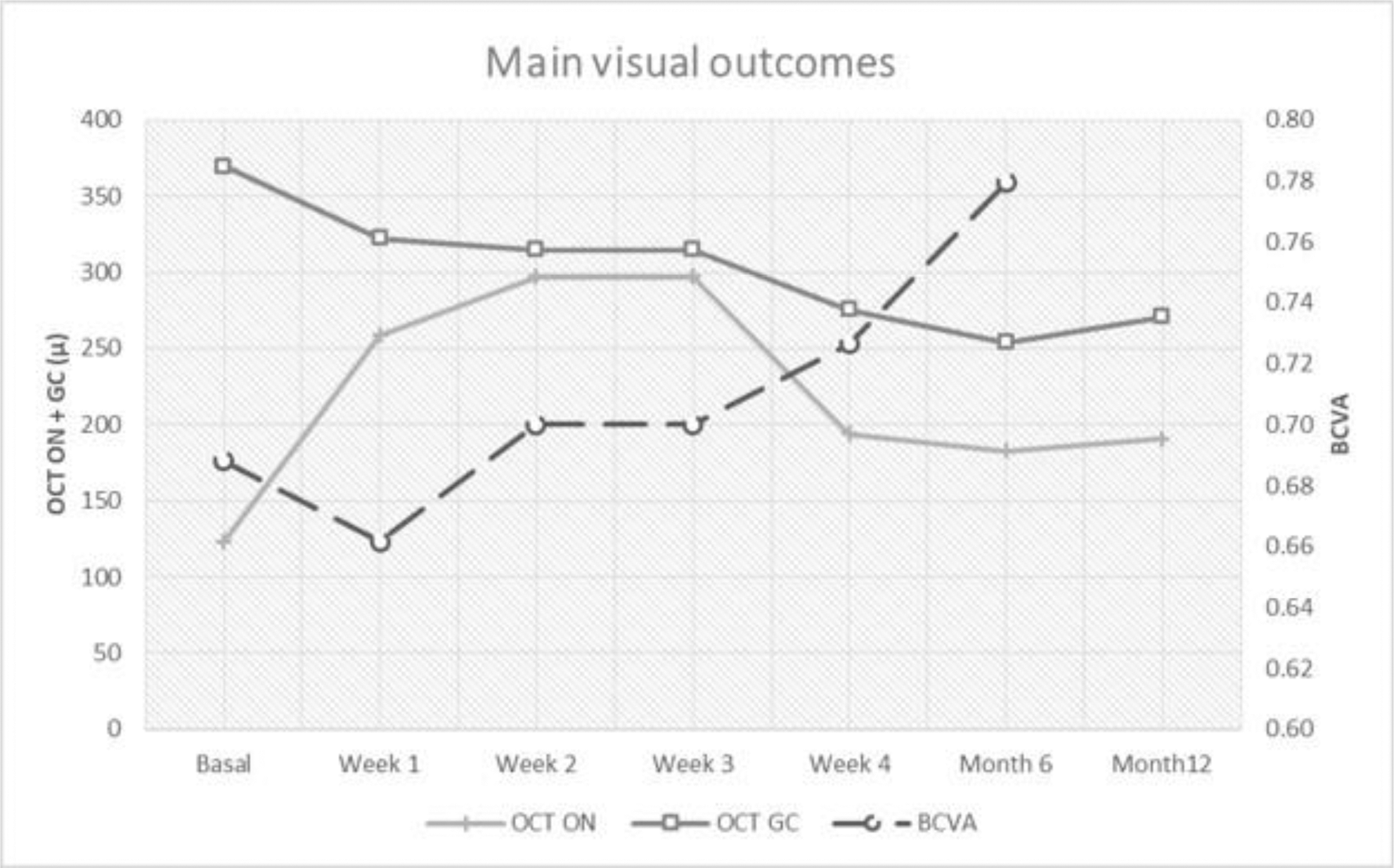
Results: We studied 19 patients (12 women/7 men); mean age of 34.8 ± 13.9 years. The underlying diseases were idiopathic (n=7), Behçet´s disease (n=5), systemic lupus erythematosus (n=2), neuromyelitis optica (n=3), sarcoidosis (n=1) and relapsing polychondritis (n=1) (TABLE). Before biological therapy and besides systemic corticosteroids, patients had received different CIS. Biological therapy was adalimumab (n=6), rituximab (n=6), infliximab (n=5) and tocilizumab (n=4). After biological therapy, an improvement in ocular parameters was observed: BCVA [0.7±0.3 to 0.8±0.3; p= 0.03], optic nerve OCT [123.2±58.3 to 190.5±175.4; p= 0.11], and ganglionar cells OCT [369.6±137.4 to 270.7±23.2; p= 0.03] at one year (FIGURE ). After a mean follow-up of 29.1 ±19.2 months, there were no severe adverse effects observed.
Conclusion: Biological therapy may be effective in patients with refractory atypical ON.
| Case | Gender/ Age | Underlying disease | Laterality | IV steroids dose (g) | Maximum prednisone oral dose (g) | Conventional immunosuppressants | Biological therapy | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| 1 | F/29 | Idiopathic | Unilateral | 4 | 60 | AZA | TCZ | No |
| 2 | F/26 | Idiopathic | Bilateral | 5.5 | 30 | AZA | TCZ | No |
| 3 | F/13 | Idiopathic | Bilateral | - | 10 | MTX | ADA | No |
| 4 | F/25 | Idiopathic | Bilateral | 4 | 60 | MTX | IFX, TCZ | No |
| 5 | F/24 | Idiopathic | Bilateral | 0.5 | 60 | MTX, AZA | ADA | No |
| 6 | M/14 | Idiopathic | Bilateral | - | 10 | MTX | ADA | No |
| 7 | F/30 | Vasculitis ANCA+ | Unilateral | 3 | 60 | AZA, MMF, LFM, CFM | RTX | Yes |
| 8 | M/21 | Behçet | Bilateral | - | 60 | MTX, AZA | ADA | Nausea Vomits |
| 9 | M/25 | Behçet | Unilateral | 0.5 | 60 | MTX, CyA | ADA | No |
| 10 | M/39 | Behçet | Unilateral | 3 | 80 | MTX, MMF | IFX | No |
| 11 | M/40 | Behçet | Unilateral | - | 80 | MMF | IFX | No |
| 12 | M/37 | Behçet | Unilateral | - | 60 | CyA | IFX | No |
| 13 | F/68 | NMO | Unilateral | 2.5 | 30 | CFM, AZA | RTX | No |
| 14 | F/41 | NMO | Unilateral | 3 | 60 | CFM | RTX | Infection |
| 15 | F/43 | NMO | Bilateral | 5 | 60 | AZA | RTX | Infusion reaction |
| 16 | F/56 | SLE | Unilateral | - | 60 | HCQ, MMF, CFM | RTX | No |
| 17 | F/47 | SLE | Unilateral | 5 | 60 | HCQ, MMF | RTX | No |
| 18 | F/43 | Relapsing polychondritis | Bilateral | 3 | 60 | MTX, CFM | IFX, TCZ | No |
| 19 | M/41 | Sarcoidosis | Bilateral | 3 | 40 | AZA | ADA | No |
Disclosure of Interests: Alba Herrero Morant: None declared, Carmen Álvarez Reguera: None declared, Vanesa Calvo del Rio Grant/research support from: MSD and Roche, Speakers bureau: Abbott, Lilly, Celgene, Grünenthal, UCB Pharma, Olga Maíz Alonso: None declared, Ana Blanco Speakers bureau: Abbvie, J. Narváez: None declared, Santos Castañeda: None declared, Esther Vicente Speakers bureau: BMS, Roche., Susana Romero-Yuste: None declared, Rosalía Demetrio-Pablo: None declared, ANA URRUTICOECHEA-ARANA: None declared, J. L. García Serrano: None declared, J. L. Callejas Rubio: None declared, Norberto Ortego: None declared, Julio Sánchez: None declared, Paula Estrada: None declared, Iñigo Rua-Figueroa: None declared, David Martínez-López: None declared, José Luis Martín-Varillas Grant/research support from: AbbVie, Pfizer, Janssen and Celgene, Speakers bureau: Pfizer and Lilly, Miguel Á. González-Gay Grant/research support from: AbbVie, MSD and Roche, Speakers bureau: AbbVie, MSD and Roche, Ricardo Blanco Grant/research support from: Abbvie, MSD and Roche, Consultant of: Abbvie, Pfizer, Roche, Bristol-Myers, Janssen and MSD, Speakers bureau: Abbvie, Pfizer, Roche, Bristol-Myers, Janssen, Lilly and MSD