

Background: Due to a high level of evidence of good test performance, accessibility, minimal invasiveness, low cost, and good overall performance, EULAR recommends ultrasound (US) of the temporal and axillary arteries as primary diagnostic imaging test in patients suspected of Giant Cell Arteritis (GCA) (1).
Despite the growing body of evidence supporting the utility of US in GCA, standardized training programs and their impact on reliability are lacking(1). In TABUL study (2), the only US study published to date using a standardised US training program, the interobserver agreement by 12 different sonographers was only moderate, illustrating the challenges presented in the education for US in GCA.
Objectives: To evaluate the impact of a standardized training program including equipment adjustment on the agreement and reliability of US in the diagnosis of GCA for experienced musculoskeletal (MSK)ultrasonographers, without previous experience on vascular US.
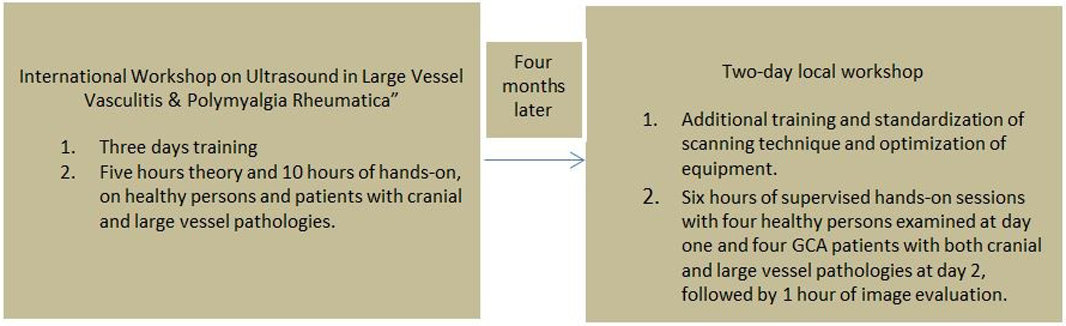
Methods: Five rheumatologists with long-standing experience in MSK US were trained by a standardized training program including equipment adjustment (Box 1) prior to a prospective, non-interventional observational study in patients suspected having GCA.The rheumatologist performing the US subsequently evaluated the images blinded to the patients data. Thereafter the images were evaluated by a blinded external expert (i.e. considered the gold standard).
Results: In three Danish centers 112 patients were included, 59% females, mean age 72.4 (SD) 7.9 years and median CRP 55 (IQR 21-100)mg/l. Median duration of prednisolone treatment prior to US examination was 0 (IQR 0 to 1) days. 92% of the patients reported a newly emerged localized headache.
The reliability between the performing ultrasonographer and the US expert for the overall GCA diagnosis, as for the diagnosis of cranial (c-GCA) and large-vessel GCA (Lv-GCA) was excellent. In addition, excellent reliability was also found for the US examination of all examined arteries (
| Variables | Pathological findings (% ) | Interobserver agreement (% ) |
Interobserver Reliability
| 95% Confidence Limits |
|---|---|---|---|---|
| US positive for GCA | 59% | 96% | 0.93 | 0.85-0.99 |
| 66/112 | ||||
| US positive for cGCA | 53% | 95% | 0.89 | 0.81-0.98 |
| 59/112 | ||||
| US positive for Lv-GCA | 19% | 96% | 0.89 | 0.78-0.995 |
| 21/112 | ||||
| Halo sign TA, all | 51% | 96% | 0.91 | 0.83-0.99 |
| segments | 57/112 | |||
| Compression sign TA, | 48% | 94% | 0.89 | 0.80-0.98 |
| 51/107 | ||||
| all segments | ||||
| Halo sign FA | 20% | 96% | 0.87 | 0.75-0.98 |
| 23/112 | ||||
| Compression sign | 16% | 96% | 0.86 | 0.73-0.99 |
| FA | 17/107 | |||
| Halo sign AA | 18% | 97% | 0.91 | 0.81-1.00 |
| 20/112 | ||||
| Halo sign AC | 4% | 100% | 1.00 | 1.00-1.00 |
| 6/11 |
TA: Temporal; FA: Facial; AA: Axillary; AC: common Carotid, artery
Conclusion: Our training program resulted in excellent reliability of US findings in patients suspected of having GCA and for the final diagnosis. The training program could be used when implementing vascular US in clinical practice.
Box 1
REFERENCES:
[1]Dejaco C et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636
[2]Luqmani R et al. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis: a diagnostic accuracy and cost-effectiveness study Health Technol Assess 2016;20:1_238
Disclosure of Interests: stavros chrysidis: None declared, Lene Terslev Speakers bureau: LT declares speakers fees from Roche, MSD, BMS, Pfizer, AbbVie, Novartis, and Janssen., Robin Christensen: None declared, Ulrich Fredberg: None declared, Knud Larsen: None declared, Tove Lorenzen: None declared, Uffe Møller Døhn: None declared, Andreas Diamandopoulos: None declared