

Background: Interferon (IFN) is known to play a role in the pathogenesis of many autoimmune diseases, among them, rheumatoid arthritis (RA). A study showed that two interferon-stimulated gene expression scores (IFN-Score-A and IFN-Score-B) can be used to predict progression to connective tissue disease (CTD) in at-risk individuals, positive for anti-nuclear antibodies (ANA+) [1]. This validated score could potentially be applied to individuals at-risk of RA.
Objectives: To investigate the role of type I IFN in patients at-risk of RA and assess its potential role as a biomarker to predict progression to inflammatory arthritis (IA).
Methods: PBMC samples were taken from 36 at-risk individuals positive for anti-citrullinated protein antibodies (ACPA+), with a non-specific musculoskeletal complaint but no clinical synovitis and a normal ultrasound scan at baseline (BL). 17 of them developed IA later on, and had a second sample taken at the moment of progression. The other 19 did not progress and had a second sample taken after 1 year. Expression of IFN stimulated genes was assessed using TaqMan. T-test was used to compare the expression of the genes at the two time points of each individual. Binary logistic regression was used to assess BL predictors of progression. Multivariable analysis was adjusted for confounders such as C-reactive protein (CRP) rheumatoid factor (RF) and ACPA titre.
Results:
Interferon stimulated genes and scores A and B
| GENE | IFN score | GENE | IFN score |
|---|---|---|---|
| CCL8 | BST2 | ||
| CEACAM1 | IFI16 | ||
| CXCL10 | IFIH1 | ||
| GBP1 | LAMP3 | ||
| IFI27 | NT5C3B | ||
| IFI44 | PHF11 | ||
| IFI44L | A | SERPING1 | B |
| IFIT1 | SOCS1 | ||
| IRF7 | SP100 | ||
| ISG15 | STAT1 | ||
| RSAD2 | TAP1 | ||
| XAF1 | TRIM38 | ||
| UBE2L6 | |||
| UNC93B1 |
Baseline characteristics and predictors of progression
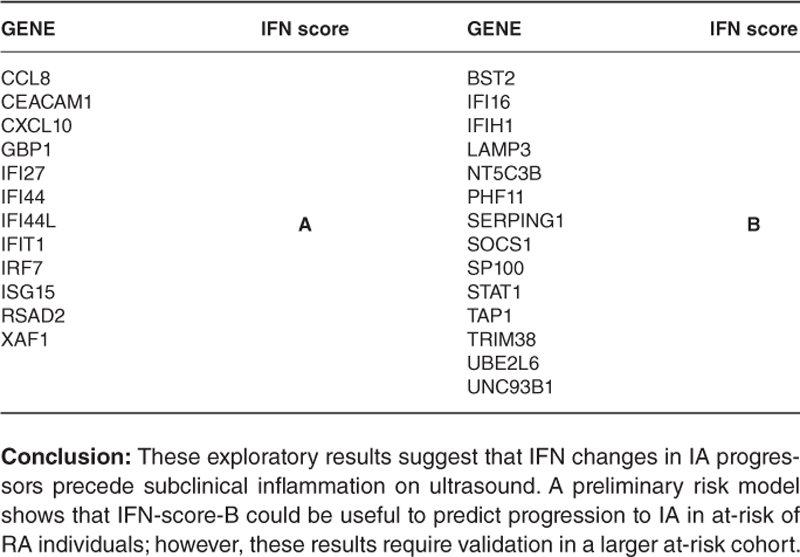
Conclusion: These exploratory results suggest that IFN changes in IA progressors precede subclinical inflammation on ultrasound. A preliminary risk model shows that IFN-score-B could be useful to predict progression to IA in at-risk of RA individuals; however, these results require validation in a larger at-risk cohort.
REFERENCES:
[1]Md Yusof MY. Ann. Rheum. Dis. 2018;77(10):1432-9.
Disclosure of Interests: Leticia Garcia-Montoya: None declared, Zoe Wigston: None declared, Agata Burska: None declared, Kulveer Mankia: None declared, Edward Vital Grant/research support from: AstraZeneca, Roche/Genentech, and Sandoz, Consultant of: AstraZeneca, GSK, Roche/Genentech, and Sandoz, Speakers bureau: Becton Dickinson and GSK, Paul Emery Grant/research support from: AbbVie, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche (all paid to employer), Consultant of: AbbVie (consultant, clinical trials, advisor), Bristol-Myers Squibb (consultant, clinical trials, advisor), Lilly (clinical trials, advisor), Merck Sharp & Dohme (consultant, clinical trials, advisor), Novartis (consultant, clinical trials, advisor), Pfizer (consultant, clinical trials, advisor), Roche (consultant, clinical trials, advisor), Samsung (clinical trials, advisor), Sandoz (clinical trials, advisor), UCB (consultant, clinical trials, advisor)