

Background: Immunogenicity related to treatment with TNF inhibitors (TNFi) is one of the causes for the decreased attainment of clinical response in patients with rheumatoid arthritis (RA) 1 . However, the involved mechanism is not entirely clear. The B-cell activating factor (BAFF) may play a role in the development of immunogenicity 2 .
Objectives: To analyse the association between baseline serum BAFF concentration and the development of immunogenicity within 6 months (m) of TNFi treatment in patients with RA.
Methods: A total of 121 patients with RA initiated at standard doses of TNFi (infliximab (n=64), adalimumab (n=13), golimumab (n=11), certolizumab (n=33)) were included in this observational study and followed for 6m. Eleven (9%) received TNFi monotherapy, 92 (65%) TNFi+MTX (methotrexate) and 31 (26%) TNFi+csDMARDs other than MTX. A cohort of 68 untreated patients with early-RA and 20 healthy individuals (age- and sex-matched with the patients) were included as controls. Serum samples were obtained at baseline and 6m, up to 24h prior to TNFi administration. The baseline serum BAFF concentration was measured using ELISA. Drug and anti-drug antibody (ADA) levels were measured using sandwich and drug-sensitive bridging ELISA, respectively. Patients were stratified according to ADA development. Depending on data distribution, comparisons were conducted using unpaired t, Mann-Whitney U or Fisher’s exact tests. The association between the development of ADA within 6m and clinical/serological variables was evaluated by uni- and multi-variable logistic regression. The presence of interactions with covariates (age, RF, ACPA, body mass index, baseline DAS28 and concomitant MTX dose) was tested, stratifying the results if this was significant (p<0.05). In case of no interaction, the model was later adjusted for these covariates.
Results: Mean serum BAFF concentration in healthy controls (373±59 pg/mL) was significantly lower compared to patients with early RA (921±388 pg/mL) or established RA (936±407 pg/mL), (p<0.0001 for both). However, BAFF concentrations did not differ between early and established RA (p=0.9).
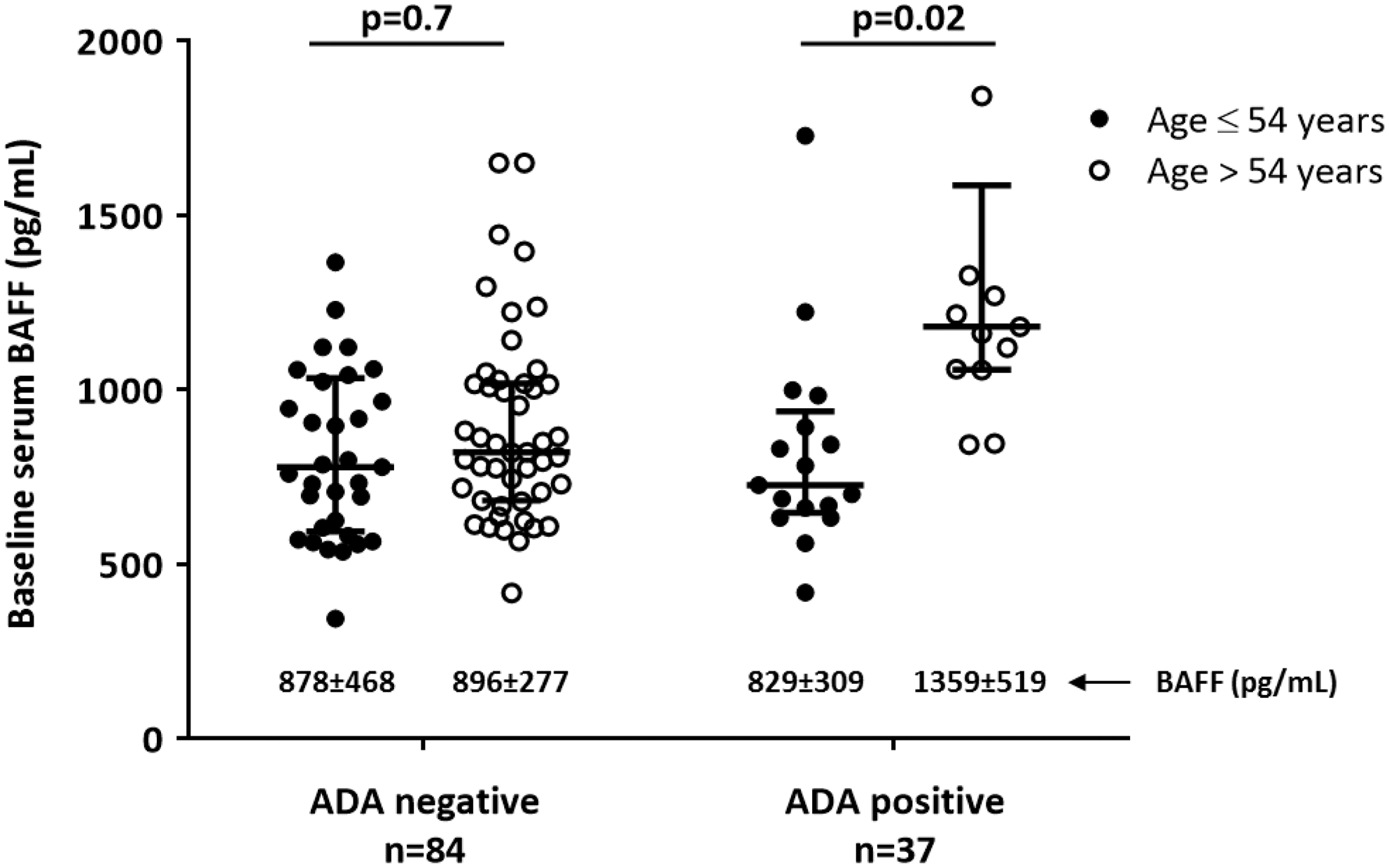
In this cohort, 37 (31%) patients developed ADA within 6m of treatment. These patients showed higher frequency of seropositivity (RF, p<0.05; ACPA<0.05), had higher baseline DAS28 (p<0.05), received lower dose of concomitant MTX (p<0.001) and had higher baseline BAFF concentration (p=0.06).The association between baseline BAFF concentration and ADA development was next investigated. The dose of concomitant MTX tended to interact on this association (p=0.06). A significant interaction was found between baseline BAFF concentration and age (Wald chi-square= 6.23; p=0.01); therefore, the results were stratified according to mean age (≤/>54yr). The results showed that baseline BAFF concentration was associated with ADA development only in older patients (OR=1.0, p=0.02); (shown in the figure). In addition, a significant Spearman correlation between baseline BAFF concentration and ADA levels at 6m was found in older patients (r=0.5, p<0.001).
Conclusion: Our results suggest that BAFF has a role in the development of immunogenicity. Nevertheless, this association depends on the age. In RA patients treated with TNFi, the development of ADA within 6m is associated with higher baseline BAFF concentration only in older patients but not in younger patients. The dose of concomitant MTX interacted on this association.
REFERENCES:
[1]Pascual-Salcedo D, et al. Rheumatology (Oxford). 2011; 50: 1445-520.
[2]Bitoun S, et al. Ann Rheum Dis. 2018; 77: 1463-70.
Acknowledgments: Nordic Pharma
Disclosure of Interests: Borja Hernández-Breijo: None declared, Ioannis Parodis: None declared, Johanna Gehin Speakers bureau: Roche, Chamaida Plasencia: None declared, Victoria Navarro-Compán Consultant of: Abbvie, Lilly, Novartis, Pfizer, UCB, Speakers bureau: AbbVie, MSD, Lilly, Novartis, Pfizer, UCB, ANA MARTÍNEZ-FEITO: None declared, David J Warren: None declared, Araceli Mezcua: None declared, Marta Novella-Navarro: None declared, Pilar Nozal: None declared, Alejandro Balsa Grant/research support from: BMS, Roche, Consultant of: AbbVie, Gilead, Lilly, Pfizer, UCB, Sanofi, Sandoz, Speakers bureau: AbbVie, Lilly, Sanofi, Novartis, Pfizer, UCB, Roche, Nordic, Sandoz