Background: Novel subcutaneous infliximab (CT-P13 SC) was developed to augment the flexibility in therapeutic use of infliximab and noninferiority (NI) of CT-P13 SC versus CT-P13 intravenous (IV) was demonstrated for efficacy in patients with rheumatoid arthritis (RA) [1]. CT-P13 SC 120mg biweekly showed consistent higher therapeutic trough levels during the treatment period, which helps in maintaining efficacy over time. Since immunogenicity has clinical importance in patients using anti-TNF alpha agents and there is a general presumption that SC route is more immunogenic than IV route, this needs careful assessment.
Objectives: Immunogenicity assessment of CT-P13 SC with further impact analysis has been performed on the pivotal data set [1] to determine whether there was any correlation between the magnitude of anti-drug antibody (ADA) positivity and clinical outcomes in RA patients.
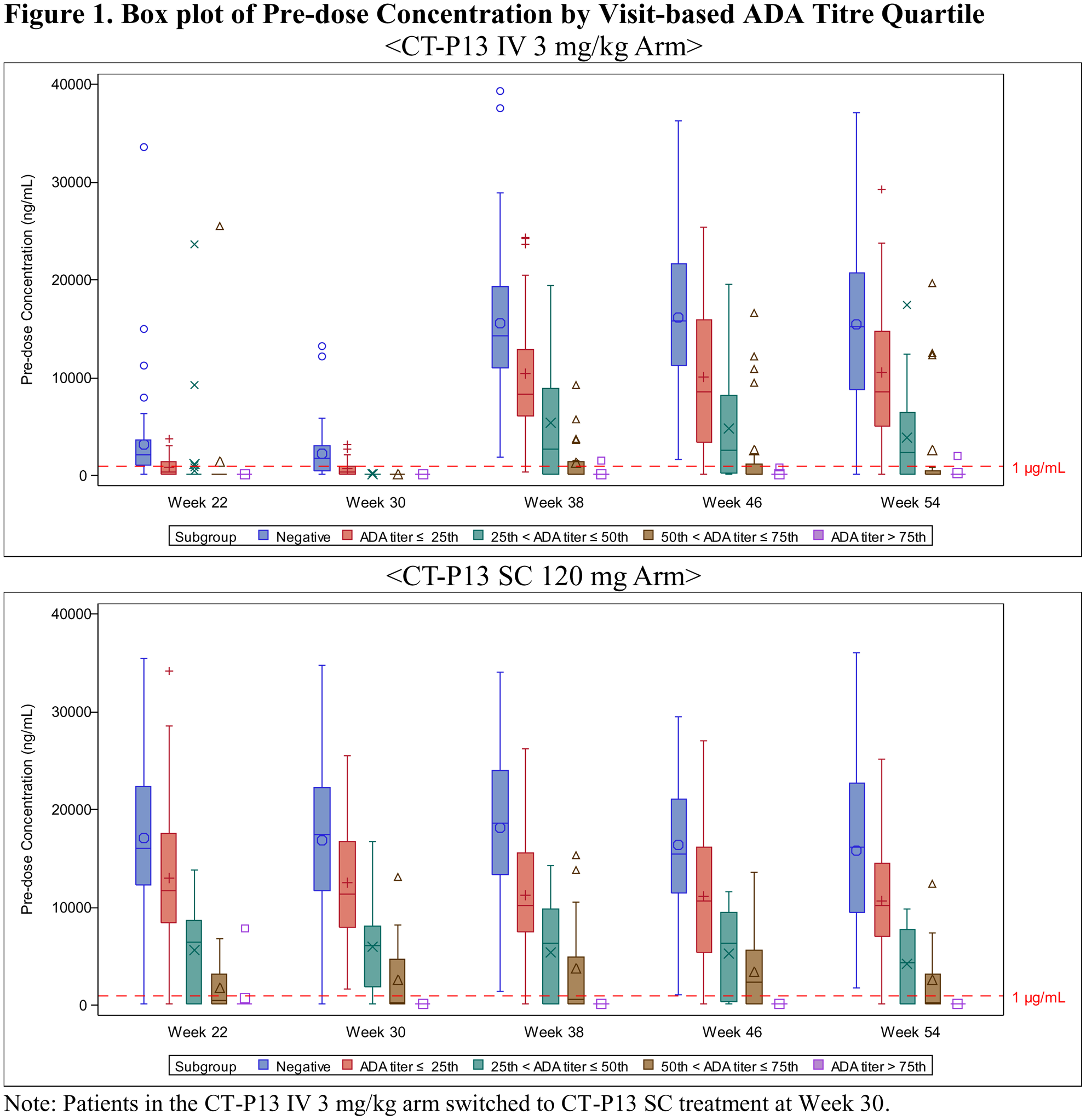
Methods: The immune response against CT-P13 in human serum was detected using an electrochemiluminescence (ECL) platform with an Affinity Capture Elution (ACE) step. An ADA ECL ACE assay showed ability to detect ADA at low levels in all samples regardless of residual drug in serum (25 ng/mL ADA in the presence of 80 μg/mL of CT-P13 in RA serum). To investigate the impact of ADA titer on PK, efficacy and safety, key clinical parameters were assessed by visit based ADA titer quartile. All patients who had ‘Positive’ ADA status result at each visit were included in the analysis and categorized into 4 groups using the 25th, 50th, 75th percentiles of ADA titer result, respectively.
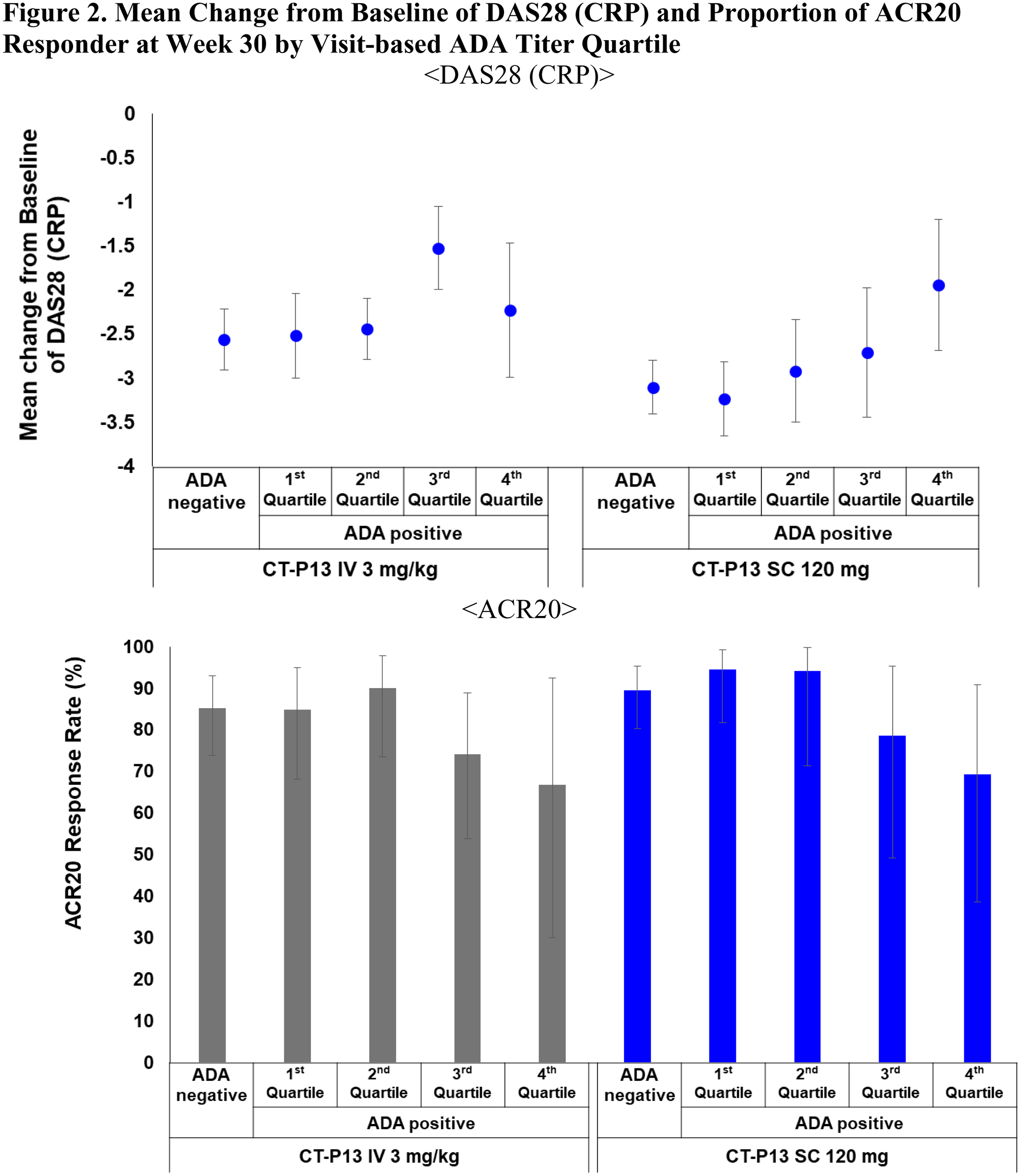
Results: The four subgroups categorized by quartiles at each visit from week 22 to week 54 were: 1st (ADA titer ≤ 3), 2nd (3 < ADA titer ≤ 9), 3rd (9 < ADA titer ≤ 27) and 4th (27 < ADA titer). There was a trend for pre-dose concentration to decrease as ADA titer increases for both CT-P13 SC and CT-P13 IV arms as expected (
Conclusion: The analysis of both ADA positivity and titer is clinically meaningful in the prediction of PK profile and clinical response. CT-P13 SC administration did not result in a greater incidence of ADA compared to the CT-P13 IV and there were no clinical differences depending on the formulation.
REFERENCES:
[1]Westhovens R, et al. Annals of the Rheumatic Diseases 2019;78:1158-1159.
Disclosure of Interests: Rene Westhovens Grant/research support from: Celltrion Inc, Galapagos, Gilead, Consultant of: Celltrion Inc, Galapagos, Gilead, Speakers bureau: Celltrion Inc, Galapagos, Gilead, DaeHyun Yoo Grant/research support from: Celltrion, Inc, Consultant of: Celltrion, Inc, Speakers bureau: Celltrion Healthcare, Inc, Piotr Wiland Grant/research support from: Celltrion, Inc, Speakers bureau: Novartis, Pfizer, Abbvie, Gedeon-Richter, Lilly, Roche, Sandoz, Marek Zawadzki Grant/research support from: Celltrion, Inc, Delina Ivanova Grant/research support from: Celltrion, Inc, Alfredo Berrocal Grant/research support from: Celltrion, Inc, Speakers bureau: Pfizer, Elias Chalouhi Grant/research support from: Celltrion, Inc, Éva Balázs Grant/research support from: Celltrion, Inc, Consultant of: Amgen, Sergii Shevchuk Grant/research support from: Celltrion, Inc, Sang Joon Lee Shareholder of: Celltrion, Inc, Employee of: Celltrion, Inc, Sung Hyun Kim Shareholder of: Celltrion, Inc, Employee of: Celltrion, Inc, JeeHye Suh Employee of: Celltrion, Inc, Chankyoung Hwang Employee of: Celltrion, Inc, Dae Seok Choi Shareholder of: Celltrion, Inc, Employee of: Celltrion, Inc