

Background: Treatment guidelines recommend early initiation of csDMARDs following diagnosis of rheumatoid arthritis (RA), with methotrexate (MTX) as first-line therapy. Scarce evidence exists on adherence to this guidance
Objectives: To characterize first-line csDMARD treatment during the first year following an RA diagnosis.
Methods: 14 real world databases (3 Primary care, 6 primary/secondary care records, 5 claims) from 9 countries were included, all mapped to the OMOP common data model.
Patients were included on the earliest event of: 1st diagnosis of RA or 1st DMARD prescription with an RA diagnosis within 30 days. Patients were >18 years-old, required 1+ year pre-index data, and at least 1-year follow-up. Study period covered 2000-2018. Previous users of DMARDs or non-RA inflammatory arthritis history were excluded. Only MTX, Hydroxychloroquine (HCQ), Sulfasalazine (SSZ) and Leflunomide (LEF) were available in all databases.
Results: We identified 323,547 eligible participants. Large variation was observed internationally (
First line csDMARD treatment during 1yr from first observed RA diagnosis
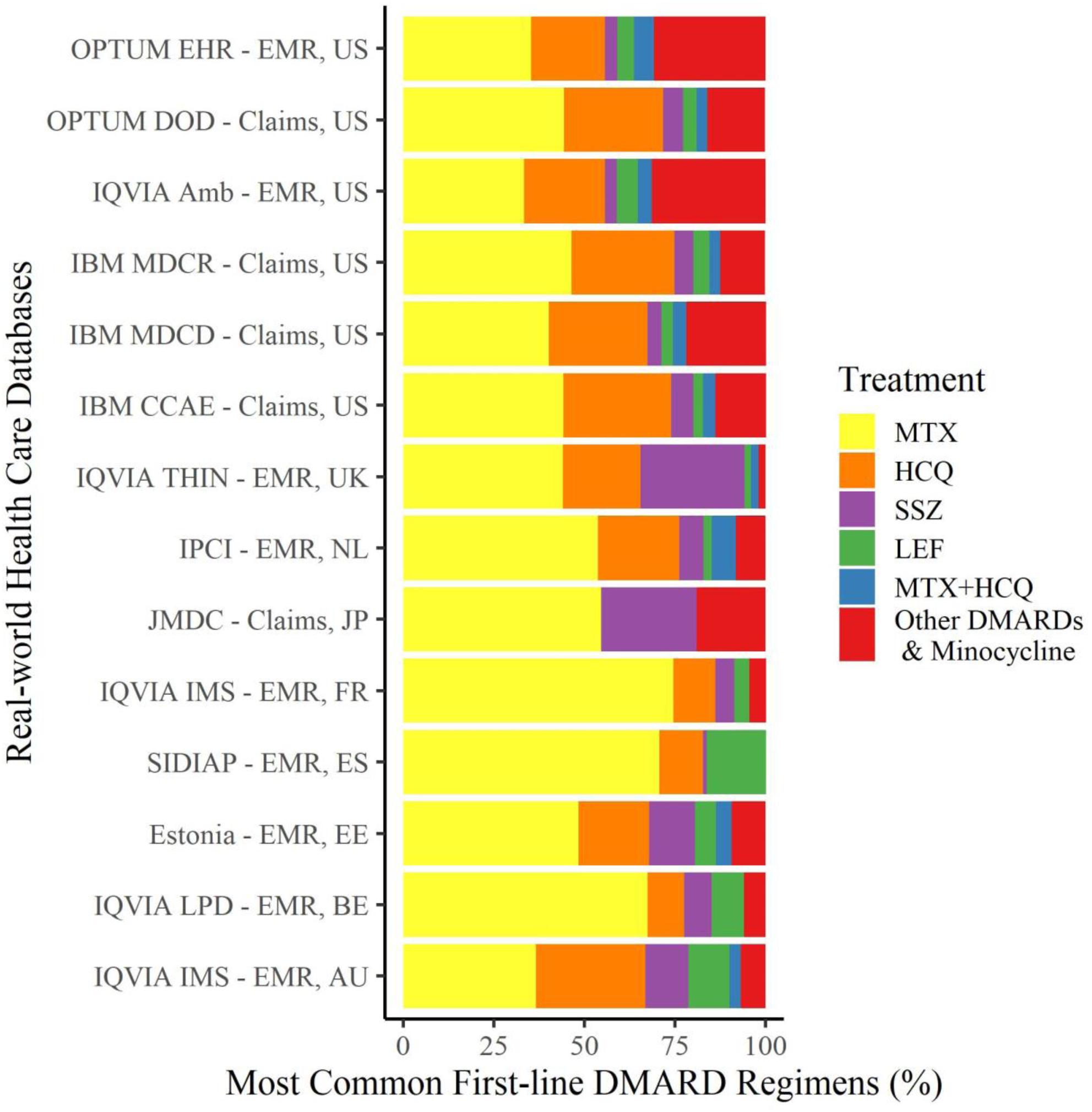
Conclusion: We report wide heterogeneity of first-line csDMARDs regimens internationally. Despite recommendations for MTX to be first line therapy, data suggest that a large proportion of patients receive alternative csDMARD.
Disclosure of Interests : Anthony G Sena Shareholder of: J&J shares, Grant/research support from: Full-time employment salary from Janssen, Consultant of: Full-time employment salary from Janssen, Employee of: Janssen employee, Paid instructor for: Janssen employee, Speakers bureau: Janssen employee, Denis Granados: None declared, Nigel Hughes Shareholder of: J&J shares, Grant/research support from: Full-time employment salary from Janssen, Consultant of: Janssen employee, Employee of: Janssen employee, Paid instructor for: Janssen employee, Speakers bureau: Janssen employee, WALID FAKHOURI Shareholder of: E Lilly Shares, Employee of: Eli Lilly and Company, Antje Hottgenroth Shareholder of: Eli Lilly shares, Employee of: Lilly Deutschland GmbH, Raivo Kolde: None declared, Sulev Reisberg: None declared, Carmen Olga Torre: None declared, Talita Duarte-Salles: None declared, Yesika Díaz: None declared, Jose Felipe Golib-Dzib Grant/research support from: Full-time employment salary from Janssen, Employee of: Yes, Janssen employee, Paid instructor for: Janssen Employee, Speakers bureau: Janssen Employee, Emily S. Brouwer Shareholder of: J&J shares, Takeda shares, Grant/research support from: Full-time employment salary from Janssen, Consultant of: Janssen employee, Employee of: Janssen employee, Paid instructor for: Janssen Employee, Speakers bureau: Janssen Employee, Edward Burn: None declared, Jennifer Lane: None declared, David Vizcaya Employee of: Bayer, Sara Bruce Wirta Employee of: Janssen-Cilag Sweden AB, Marcel de Wilde: None declared, Katia Verhamme: None declared, Peter Rijnbeek: None declared, Elke Theander Employee of: Janssen-Cilag Sweden AB, Katerina Chatzidionysiou Consultant of: AbbVie, Pfizer, Lilly., Daniel Prieto-Alhambra Grant/research support from: Professor Prieto-Alhambra has received research Grants from AMGEN, UCB Biopharma and Les Laboratoires Servier, Consultant of: DPA’s department has received fees for consultancy services from UCB Biopharma, Speakers bureau: DPA’s department has received fees for speaker and advisory board membership services from Amgen, Patrick Ryan: None declared