

Background: The treat-to-target Care in Early Rheumatoid Arthritis (CareRA) trial demonstrated that remission induction with csDMARD combinations and step-down glucocorticoids (GCs) was not superior over methotrexate (MTX) monotherapy with step-down GCs (Cobra Slim) in RA patients with a high-risk profile (1). Moreover, Cobra Slim showed benefit over a tight step-up with MTX in monotherapy (TSU) in RA patients with a low-risk profile.
Objectives: To compare the long term outcomes up to 5 years of different initial intensive treatment strategies in participants of the CareRA-plus study.
Methods: In the CareRA trial, patients with DMARD naïve early RA were stratified in a high- or low-risk group based upon the presence of serummarkers, disease activity and erosive status. High-risk patients were randomised to Cobra Classic (MTX+sulphasalazine with highly dosed GC remission induction scheme), Cobra Avant-Garde (MTX+leflunomide with moderately dosed GC scheme) or Cobra Slim. Low-risk patients were randomised to Cobra Slim or TSU. Patients completing this trial were eligible for the CareRA-plus observational study. Here, patients were evaluated 6-monthly over 3 years. Therapy adaptation was left to the treating physician. Efficacy was assessed by DAS28-CRP and HAQ and compared between the originally allocated treatment arms. The 5-year evolution from CareRA baseline of DAS28-CRP and HAQ was assessed via linear mixed models. All adverse events (AEs), considered to be clinically relevant by investigators, and DMARD/GCs therapy were registered.
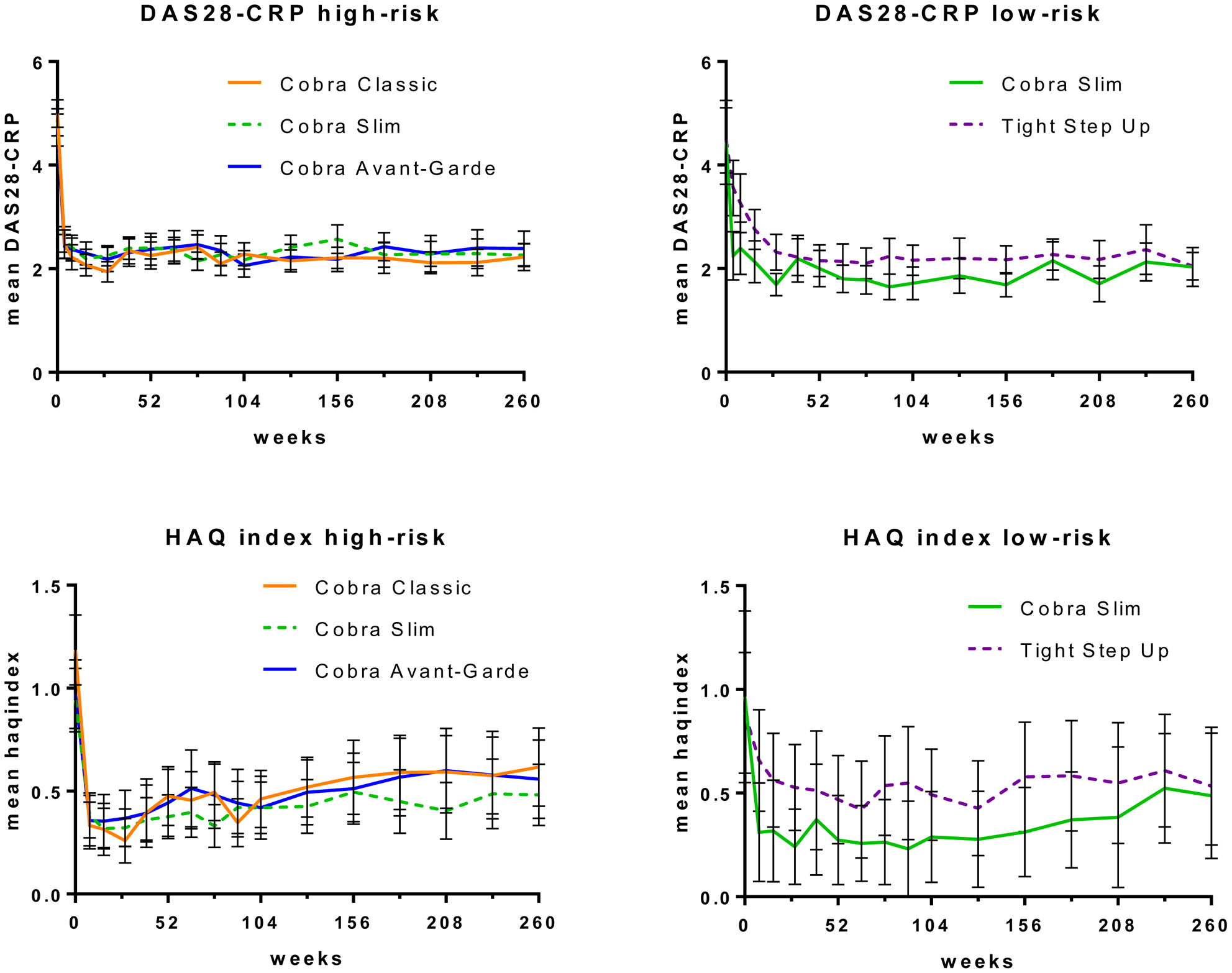
Results: Of 322 eligible patients, 252 (78%) were included in CareRA-plus, of which 203 (81%) completed the study. Characteristics and outcomes at the CareRA closing visit (year 2) did not differ between patients entering CareRA-plus or not. DAS28-CRP<2.6 at year 5 in high-risk patients was 72%, 77% and 64% in the Classic, Slim and Avant-Garde group respectively (p=0.403). In the longitudinal analyses, all treatment arms in the high-risk group had comparable DAS28-CRP (p=0.921) and HAQ scores over time (p=0.540). In the low-risk population, 83% of patients in the Slim and 82% in the TSU arm had DAS28-CRP<2.6 at year 5 (p=0.945). Low-risk patients starting Cobra-Slim had lower DAS28-CRP scores over 5 years than those receiving TSU (p= 0.002). HAQ score over time did not differ (p=0.129). In high-risk patients, the total numbers of AEs throughout CareRA-plus, were 70 in 36 Classic, 95 in 48 Slim and 80 in 36 Avant-Garde patients (p=0.182). In the low-risk group there were 18 AEs in 10 Slim and 36 in 17 TSU patients (p=0.048). During the 5-year study, biologics were initiated in 22% of all patients: 23% of Classic, 23% of Slim high-risk, 25% of Avant-Garde, 17% of Slim low-risk, and 15% of TSU patients. At the year 5 visit, 71%, 61% and 50% of high-risk patients were on csDMARD monotherapy (mostly MTX) in Classic, Slim and Avant-Garde respectively. Of the low-risk group, 65% in COBRA-Slim and 62% in TSU were taking a single csDMARD. At the year 5 visit, 9% of all participants received chronic oral GC therapy (>3 months).
Conclusion: All intensive treatment strategies resulted in excellent long-term clinical outcomes. Initial Cobra Slim therapy showed comparable 5-year effectiveness as Cobra Classic and Avant-Garde in high-risk early RA patients and better efficacy and safety than conservative step up treatment in low-risk patients.
Mean disease activity by DAS28-CRP or mean functionality by HAQ index scores for high-risk or low-risk patients.
REFERENCES:
[1]Stouten, V. et al. Effectiveness of different combinations of DMARDs and glucocorticoid bridging in early rheumatoid arthritis: two-year results of CareRA. Rheumatology (Oxford). (2019) doi: 10.1093/rheumatology/kez213.
Disclosure of Interests : Veerle Stouten: None declared, Rene Westhovens Grant/research support from: Celltrion Inc, Galapagos, Gilead, Consultant of: Celltrion Inc, Galapagos, Gilead, Speakers bureau: Celltrion Inc, Galapagos, Gilead, Diederik De Cock: None declared, Sofia Pazmino: None declared, Johan Joly: None declared, Delphine Bertrand: None declared, Kristien Van der Elst: None declared, Patrick Verschueren Grant/research support from: Pfizer unrestricted chair of early RA research, Speakers bureau: various companies