

Background: Upadacitinib (UPA) is an oral JAK inhibitor approved for the treatment of rheumatoid arthritis (RA). The background rate of herpes zoster (HZ) in patients (pts) with RA is around 0.98/100 person years (PY) 1 . Pts with RA receiving JAK inhibitors have been reported to have an increased risk of HZ.
Objectives: To evaluate the incidence and risk factors for HZ in pts with RA receiving UPA relative to active comparators in the Phase 3 clinical trial program.
Methods: The incidence rate of HZ was determined in pts receiving UPA (as monotherapy [mono] or combination therapy) in five randomized Phase 3 trials (SELECT-EARLY, SELECT-MONOTHERAPY, SELECT-NEXT, SELECT-COMPARE, and SELECT-BEYOND), of which 4 evaluated both the UPA 15 and 30 mg once-daily (QD) doses and 1 trial (SELECT-COMPARE) evaluated only the 15 mg QD dose. Incidence of HZ was also determined in pts receiving adalimumab (ADA) + methotrexate (MTX) in SELECT-COMPARE and MTX mono in SELECT-EARLY. Risk factors for HZ were assessed using univariate and multivariate Cox regression models. Data cut-off was 30 June 2019.
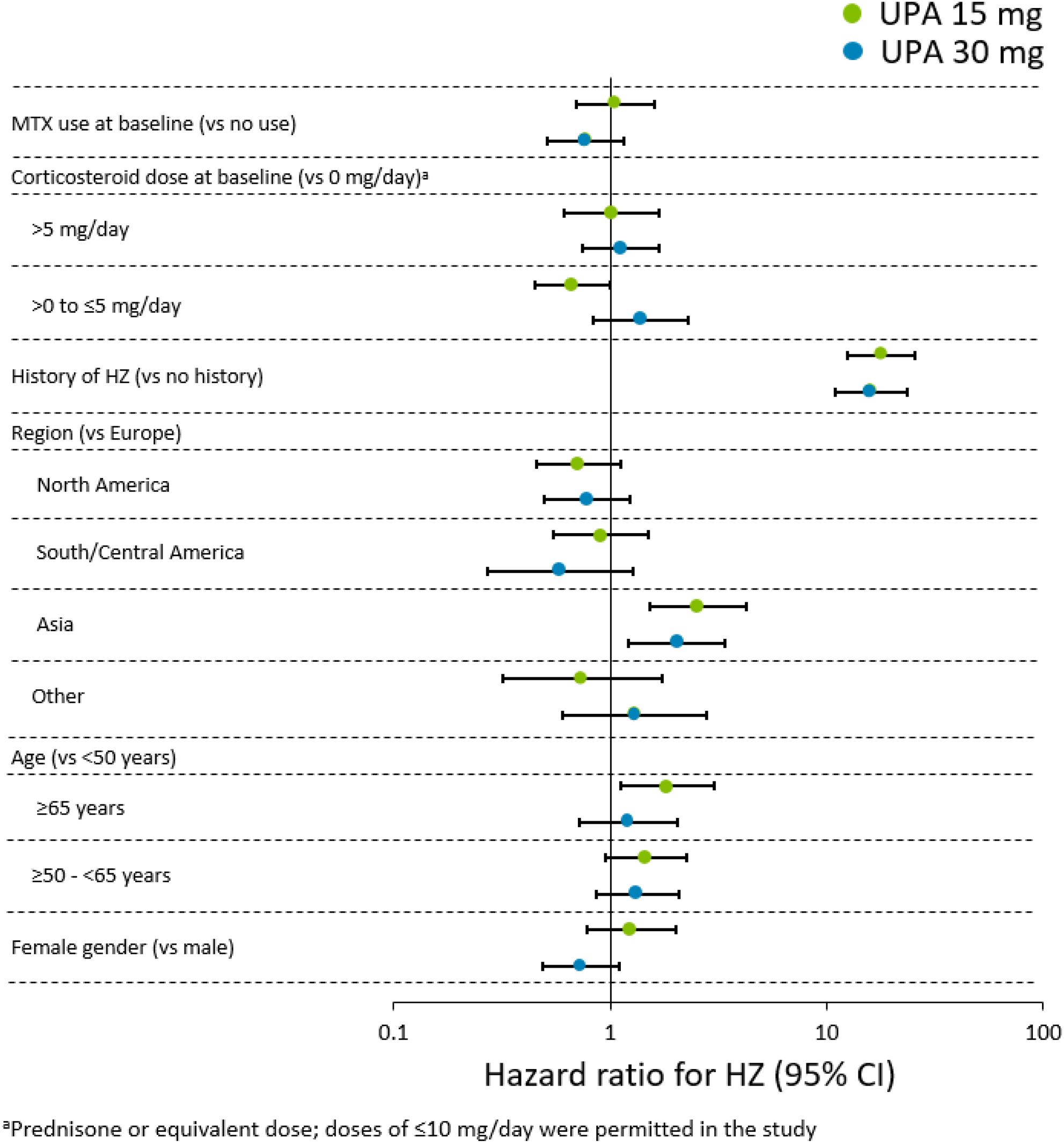
Results: Overall, 2629 pts who received UPA 15 mg QD (4565.8 patient-years [PY]), 1204 pts who received UPA 30 mg QD (2309.7 PY), 579 pts who received ADA + MTX (768.6 PY), and 314 pts who received MTX mono (456.0 PY) were analyzed. Fewer than 5% of pts across the treatment groups reported prior HZ vaccination. HZ (n/100 PY [95% CI]) occurred in 142 pts (3.1 [2.6–3.7]) with UPA 15 mg, 126 pts (5.5 [4.5–6.5]) with UPA 30 mg, 8 pts (1.0 [0.4–2.1]) with ADA + MTX, and 5 pts (1.1 [0.4–2.6]) with MTX mono. Most of the HZ cases (~71%) with UPA ( Table ) and all cases with ADA + MTX and MTX mono involved a single dermatome. Ophthalmic involvement was seen in 6 (4.2%) and 3 (2.4%) cases in the UPA 15 and 30 mg groups, respectively, and unilateral involvement with multiple dermatomes was seen in 26 (18.3%) and 23 (18.3%) cases. There was a single case of HZ meningitis reported in a Japanese pt on UPA 30 mg. In multivariate analyses, prior history of HZ and Asian region were associated with an increased risk of HZ in both the UPA groups (p≤0.01; Figure ). In addition, pts ≥65 years old had increased risk of HZ in the 15 mg group.
Conclusion: HZ events in pts with RA receiving UPA were more common in the 30 mg vs 15 mg group, and in both UPA groups compared with the ADA + MTX and MTX groups.
REFERENCES:
[1]Smitten AL, et al. Arthritis Rheum 2007;57:1431–8
Summary of extent of involvement in pts with HZ
| Categories, n (% ) a |
Any UPA 15 mg QD
|
Any UPA 30 mg QD
|
|---|---|---|
| Total patients with ≥1 HZ event | 142 (5.4) | 126 (10.5) |
| Single dermatome | 101 (71.1) | 89 (70.6) |
| Ophthalmic involvement | 6 (4.2) | 3 (2.4) |
| HZ Oticus (Ramsay Hunt Syndrome) | 2 (1.4) | 1 (0.8) |
| Multidermatomal (unilateral) b | 26 (18.3) | 23 (18.3) |
| Disseminated, cutaneous only (no CNS involvement) c | 7 (4.9) | 8 (6.3) |
| Disseminated with CNS or visceral involvement | 0 | 1 (0.8) d |
| Missing | 8 (5.6) | 5 (4.0) |
a Pts may fall into >1 category; b ≤2 adjacent dermatomes; c ≥3 dermatomes, unilateral nonadjacent dermatomes, or bilateral dermatomes; d HZ meningitis
Multivariable-adjusted risk factors for HZ in pts receiving UPA
Disclosure of Interests : Kevin Winthrop Grant/research support from: Bristol-Myers Squibb, Consultant of: AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, GSK, Pfizer Inc, Roche, UCB, Peter Nash Grant/research support from: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Speakers bureau: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, UCB, Kunihiro Yamaoka Speakers bureau: AbbVie GK, Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd, Mitsubishi-Tanabe Pharma Corporation, Pfizer Japan Inc., and Takeda Pharmaceutical Company Ltd, Eduardo Mysler Grant/research support from: AbbVie, Lilly, Pfizer, Roche, BMS, Sandoz, Amgen, and Janssen., Consultant of: AbbVie, Lilly, Pfizer, Roche, BMS, Sandoz, Amgen, and Janssen., Leonard Calabrese Consultant of: AbbVie, GSK, Bristol-Myers Squibb, Genentech, Janssen, Novartis, Sanofi, Horizon, Crescendo, and Gilead, Speakers bureau: Sanofi, Horizon, Crescendo, Novartis, Genentech, Janssen, and AbbVie, Nasser Khan Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Jose Jeffrey Enejosa Shareholder of: AbbVie, Employee of: AbbVie, Yanna Song Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Jessica Suboticki Shareholder of: AbbVie Inc., Employee of: AbbVie Inc., Jeffrey R. Curtis Grant/research support from: Abbvie, Amgen, BMS, Corrona, Crescendo, Janssen, Pfizer, Regeneron/Sanofi, and UCB, Consultant of: AbbVie, Amgen, BMS, Corrona, Crescendo, Janssen, Pfizer, Sanofi/Regeneron, and UCB