

Background: A study of 15 PSS patients showed that leflunomide had no significant effect on the decrease of salivary flow rate and the formation of lymphocytic infiltrates in salivary glands [1] . However, due to the small number of samples included in this study and the small size of human salivary gland biopsies, the therapeutic effect of leflunomide may be underestimated. At present, there is no further study on this issue, the effect of leflunomide on Sjogren’s syndrome is still not clear.
Objectives: To investigate the therapeutic effect of leflunomide on salivary gland secretion dysfunction in the NOD mice with Sjogren’s syndrome.
Methods: The NOD mice were randomly divided into four groups: preventive drug group, preventive control group, therapeutic drug group, and therapeutic control group. Salivary flow rate was measured after pilocarpine stimulation; After hematoxylin and eosin staining, the average number and area of infiltrating lesions were compared; The percentage of CD3 + T, CD4 + T, CD8 + T, CD44 + CD62L - CD4 + T, CD19 + B, and CD138 + B cells in submandibular gland and spleen were detected by flow cytometry; The levels of serum inflammatory factors TNF-a, IL-17A and IL-6 were detected by CBA method.
Results: The salivary flow rate (t = -5.81, P<0.001; z =-3.61,P<0.05), the number of infiltrating foci(t=3.95,P<0.01; t=4.94,P<0.001)and the average area of infiltrating foci(t=3.18.61,P<0.05; z=2.35,P<0.05)in the treatment groups were significantly ameliorated. CD4 + T cells(t=2.39 P<0.05; t´=3.82 P<0.01)and CD44 + CD62L - CD4 + T cells(t´=3.53,P<0.05; t´=3.36,P<0.05)in the submandibular gland were significantly decreased. CD3 + T(t=6.08, P<0.001; t=2.76,P<0.05),CD4 + T(t´=3.73,P<0.05; t=2.39, P<0.05), CD19 + B(t=5.88,P<0.001; t´=4.23, P<0.01) and CD138 + B (t=4.30, P<0.001; t=4.46, P<0.01) cells in the spleen were also significantly decreased. In addition, the serum IL-17A of the treatment group reduced to lower level(t=4.15,p<0.01;t=3.36,p<0.01),and the TNF-a level of the preventive drug group decreased(t=4.56; p<0.001).
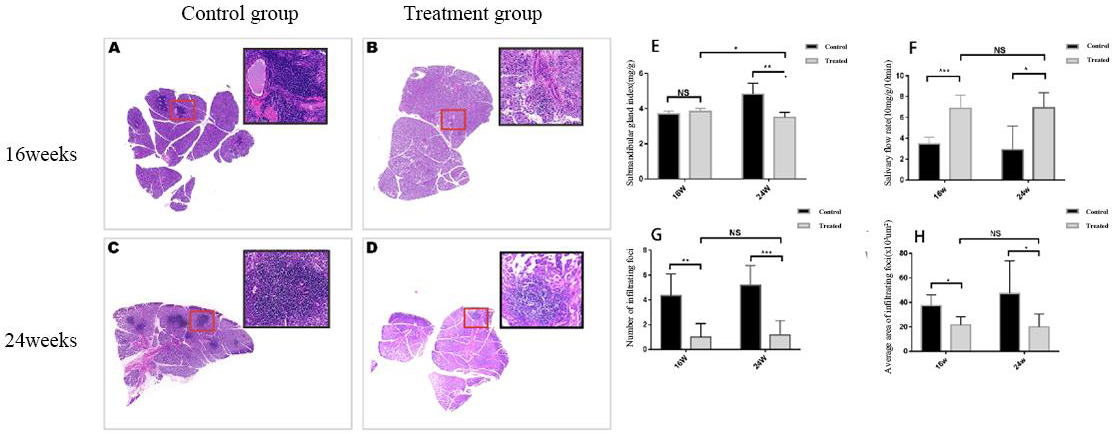
Leflunomide reduced lymphocyte infiltration and improved salivary gland function in NOD mice. A-D: Histology of the submandibular glands of NOD mice in control and treated groups. E-H: The comparison of the submandibular gland index, salivary flow rate, number of infiltrating foci and average area of the submandibular gland, between the control and treated groups. (mean±SD; n=7 per group; * P<0.05, ** P<0.01, *** P<0.001).
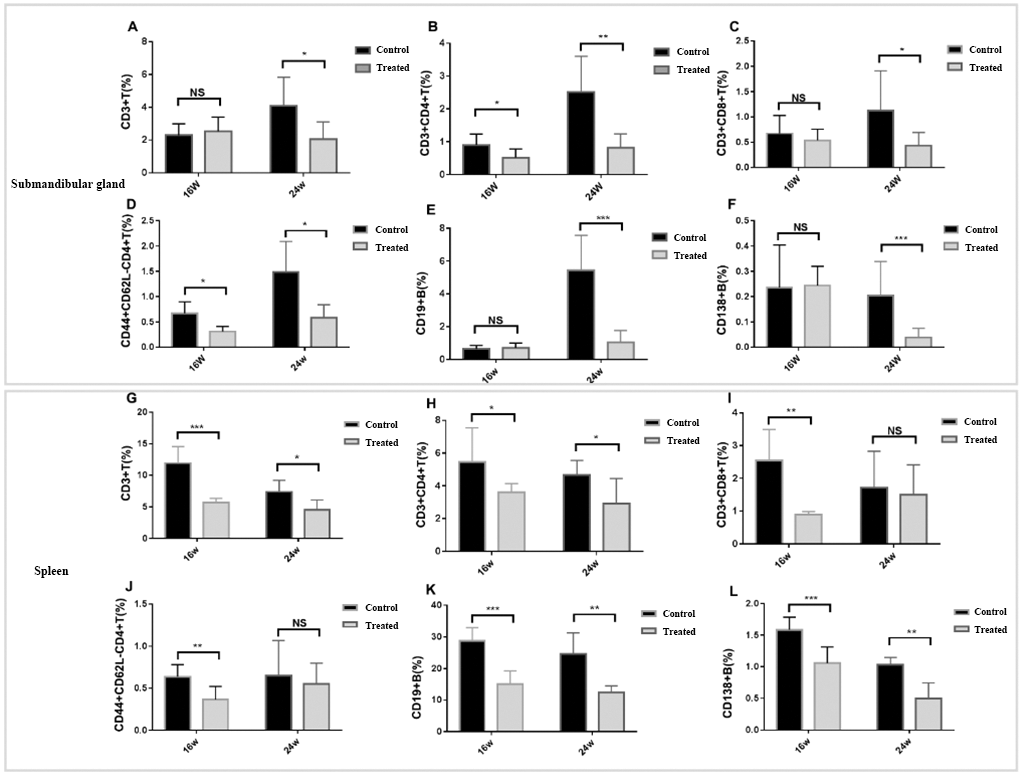
Changes of lymphocyte subsets in submandibular gland and spleen A-L: Frequencies of CD3+ T cells, CD4+ T cells, CD8+ T cells, CD44+ CD62L-CD4+T cells, CD19+ T cells and CD138+ B cells in the SMG tissues and the spleen collected from treated group and control group. (mean±SD; n=7 per group; * P<0.05, ** P<0.01, *** P<0.001).
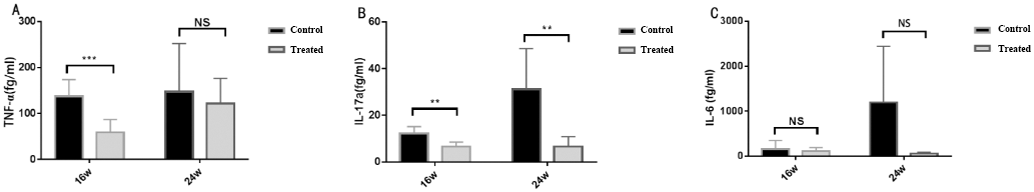
The proinflammatory cytokine levels of TNF-α, IL-17, and IL-6 in the serum samples from the treated and control group. (mean±SD; n=7 per group; * P<0.05, ** P<0.01, *** P<0.001).
Conclusion: Leflunomide may prevent and improve salivary gland hypofunction and inhibit immune activation in NOD mice, providing a theoretical basis for evaluating leflunomide in the treatment of Sjogren’s syndrome.
REFERENCES:
[1]Van Woerkom JM, Kruize A A, Geenen R, et al. Safety and efficacy of leflunomide in primary Sjögren’s syndrome: a phase II pilot study [J]. Ann. Rheum. Dis, 2007, 66(8): 1026-32.
DOI: 10.1136/ard.2006.060905.
Acknowledgments : The authors thank the Center for Scientific Research of Anhui Medical University for valuable help in our experiment.
Disclosure of Interests : None declared