

Background: Acute anterior uveitis (AAU), inflammation of the anterior uveal tract, is reported in up to 40% of patients (pts) with axial spondyloarthritis (axSpA). 1 AAU is associated with significant clinical burden; symptoms include blurred vision, photophobia and pain. 2 Previous studies have shown that TNF inhibitors (TNFi) can reduce AAU flare incidence in pts with radiographic axSpA, 3-5 but few have focused on pts across the full axSpA spectrum.
Objectives: To analyse the impact of certolizumab pegol (CZP) treatment on AAU in pts with active radiographic and non-radiographic axSpA and a recent history of AAU.
Methods: C-VIEW (NCT03020992) is an ongoing multicentre, open-label, phase 4 study. Pts had active axSpA according to the ASAS classification, a history of recurrent AAU (≥2 AAU flares in total and ≥1 AAU flare in the year prior to study entry), were HLA-B27 positive, and were eligible for TNFi treatment (previous failure of ≥2 NSAIDs, biologic naïve or had failed ≤1 TNFi). Pts received CZP 400 mg at Weeks (Wks) 0/2/4, then 200 mg every two wks (Q2W) to Wk 96. The primary variable was incidence of AAU flares compared to historic rates. A pre-specified interim analysis compared AAU incidence in the 48 wks prior to CZP treatment with the 48 wks of treatment, using Poisson regression adjusted for possible within-pt correlations, with period (pre- and post-baseline) and axSpA disease duration as covariates. Incidence rates (IR) were calculated based on the number of cases/pts at risk over 48 wks. Observed data are reported.
Results: Of 115 enrolled pts, 89 initiated CZP treatment; 85 completed Wk 48. Baseline characteristics are shown in the
Baseline characteristics
| CZP 200 mg Q2W (N=89 ) | |
|---|---|
| Age (years), mean ± SD | 46.5 ± 11.2 |
| Male, n (%) | 56 (62.9) |
| Racial group, n (%) | |
| Caucasian | 87 (97.8) |
| Other | 2 (2.2) |
| Diagnosis, n (%) | |
| Radiographic axSpA | 76 (85.4) |
| Non-radiographic axSpA | 13 (14.6) |
| Duration of axSpA (years), mean ± SD | 8.6 ± 8.4 |
| Time since onset of first uveitis flare (years), mean ± SD | 9.9 ± 9.0 |
| ASDAS, mean ± SD | 3.5 ± 0.9 |
| BASDAI, mean ± SD | 6.5 ± 1.5 |
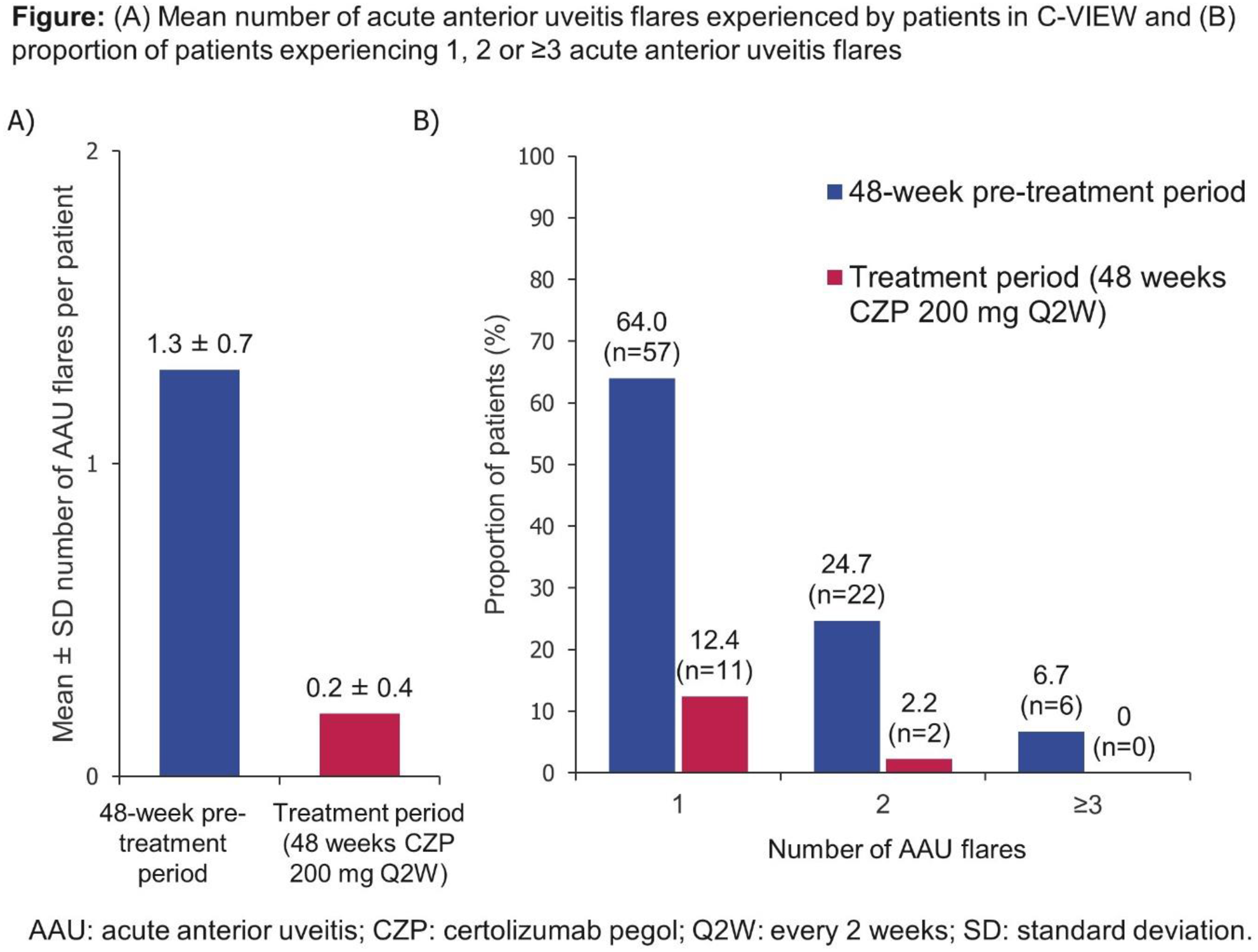
Conclusion: In this open-label study, AAU flare rate significantly reduced in axSpA pts with a history of recurrent AAU during the first 48 wks of CZP. Pts also experienced substantial improvements in axSpA disease activity.
REFERENCES:
[1]Martin TM. Curr Opin Rheumatol 2002;14:337–41
[2]Bacchiega ABS. Rheumatology (Oxford) 2017;56:2060–7
[3]van der Heijde D. Rheumatology (Oxford) 2017;56:1498–509
[4]van Bentum RE. J Rheumatol 2019;46:153–9
[5]van Denderen JC. J Rheumatol 2014;41:1843–8
Acknowledgments: This study was funded by UCB Pharma. Editorial services were provided by Costello Medical.
Disclosure of Interests: Irene van der Horst-Bruinsma Grant/research support from: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Consultant of: AbbVie, Novartis, Eli Lilly, Bristol-Myers Squibb, MSD, Pfizer, UCB Pharma, Rianne van Bentum: None declared, Frank Verbraak Grant/research support from: Bayer, Novartis, IDxDR, UCB Pharma, Consultant of: Bayer, Novartis, IDxDR, UCB Pharma, Thomas Rath Grant/research support from: AbbVie, Bristol-Myers Squibb, Chugai, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB Pharma, James Rosenbaum Consultant of: AbbVie, Corvus, Eyevensys, Gilead, Novartis, Janssen, Roche, UCB Pharma; royalties from UpToDate, Maria Misterska-Skora: None declared, Bengt Hoepken Employee of: UCB Pharma, Oscar Irvin-Sellers Employee of: UCB Pharma, Brenda VanLunen Employee of: UCB Pharma, Lars Bauer Employee of: UCB Pharma, Martin Rudwaleit Consultant of: AbbVie, BMS, Celgene, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, UCB Pharma