

Objectives: Secukinumab (SEC) is an interleukin-17 inhibitor used to treat patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA). Drug maintenance is often used as a proxy for treatment effectiveness and safety in real life settings. We aim to assess SEC maintenance in routine clinical practice and to identify survival predictors associated.
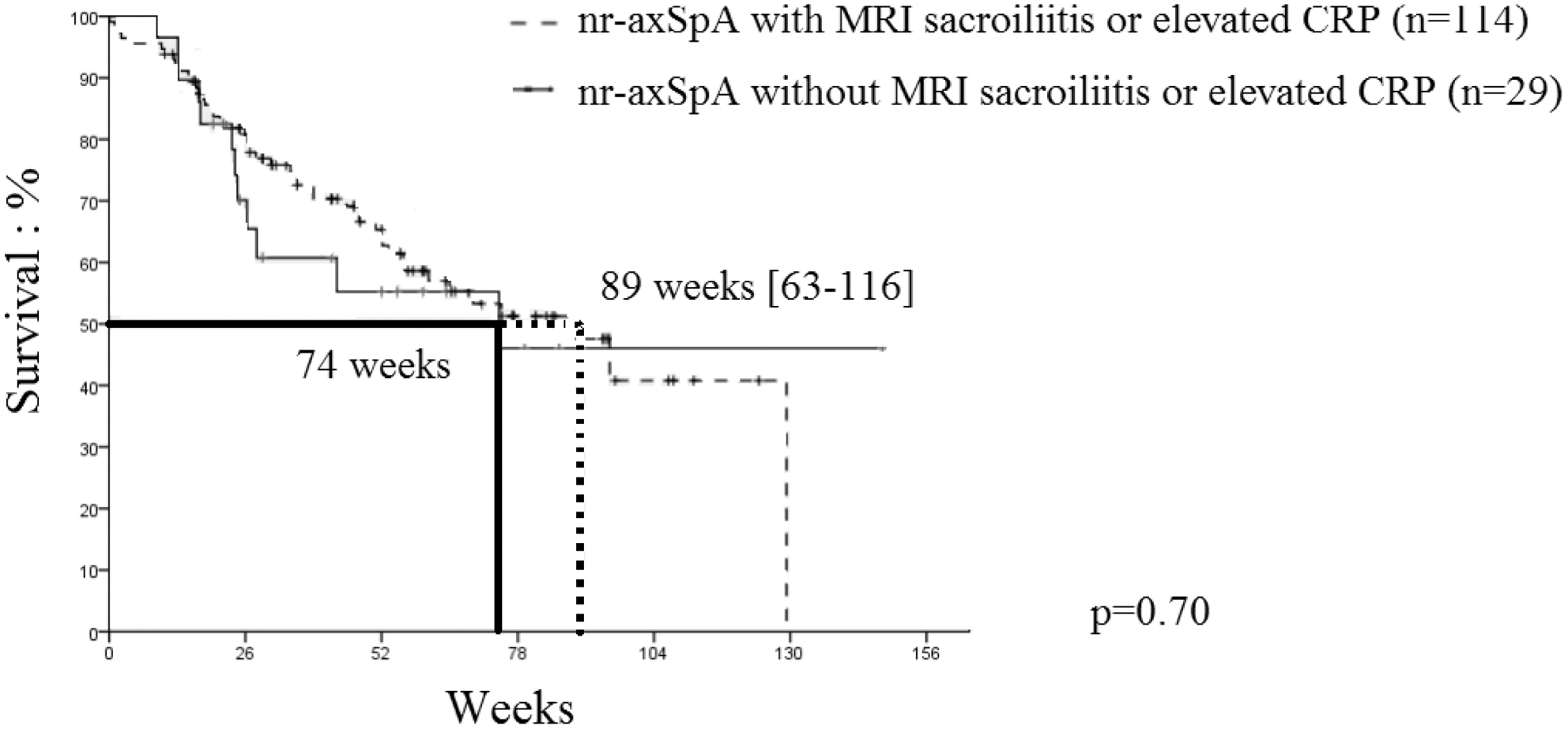
Methods: We conducted a retrospective, longitudinal, observational, multicenter study including all patients (pts) with axSpA or PsA who received at least 1 injection of SEC between July 2016 and October 2019. We collected patient’s demographics and clinic characteristics, SEC date of initiation and dosage and dosage modification of SEC, previous biologic Disease-modifying antirheumatic drugs (bDMARDs) and concomitant treatments. Date and reasons of discontinuation – i.e., lack of efficacy, safety issue, sustained remission or others – were collected. Several potential maintenance predictors were tested: age, gender, disease (axSpA or PsA), smoking status, bDMARDs history and concomitant treatment. Among patients with non-radiographic axSpA (nr-axSpA), evidence of MRI sacroiliitis or elevated CRP were also assessed as potential maintenance predictors. Drug maintenance was analyzed by the Kaplan-Meier method and adjusted for baseline factors were estimated by log rank analysis.
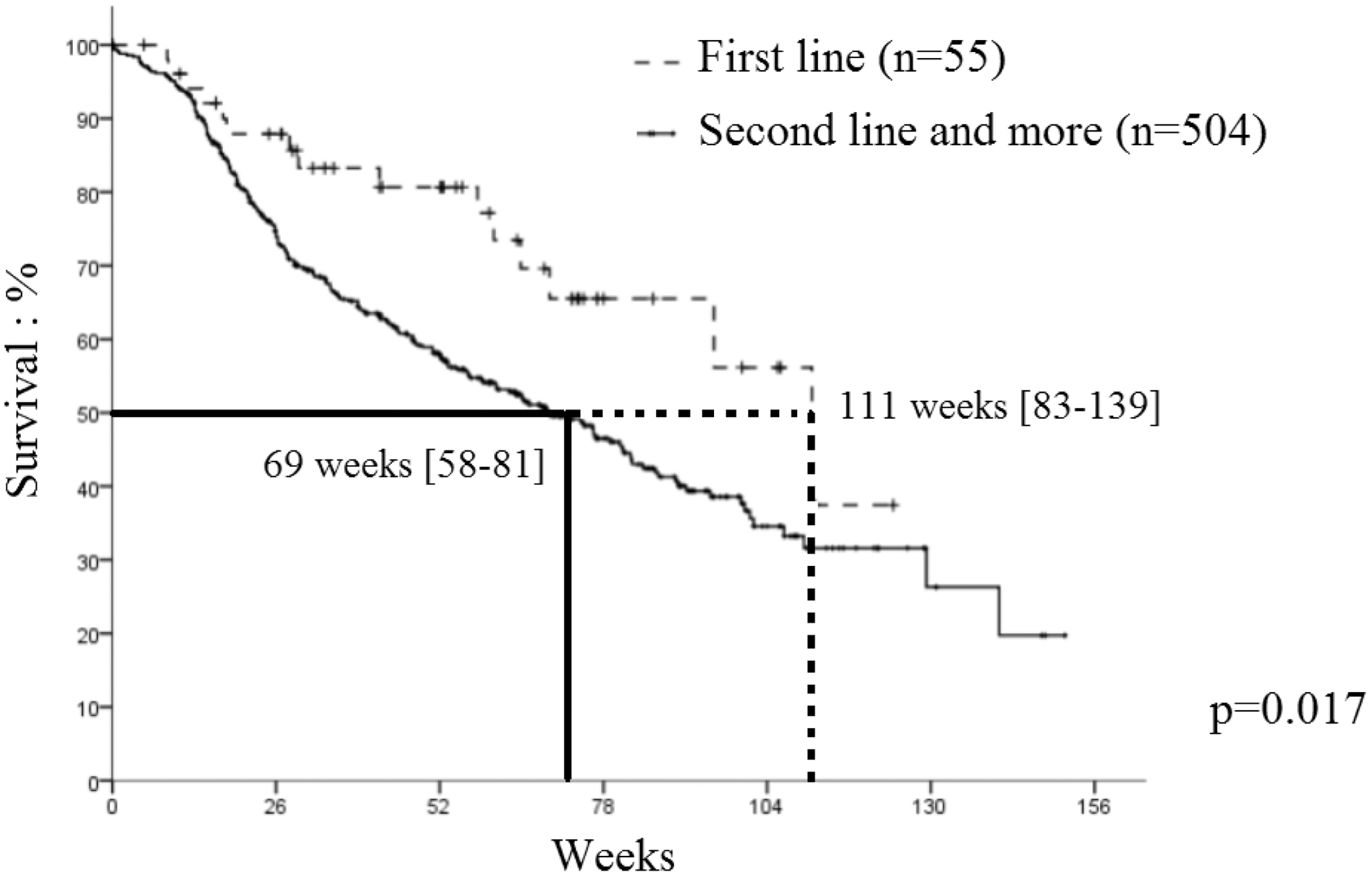
Results: The main characteristics of the 561 pts included were the following: 363 (64.7%) axSpA, 198 (35.3%) PsA, 329 (58.6%) female, mean age 45,6 +/- 12 years, 221 (39.4%) smokers, 175 (31.2%) radiographic sacroiliitis, 259 (46.2%) MRI sacroiliitis, 198 (35.3%) elevated CRP, 247 (44.0%) HLA B27 positive, mean BASDAI 48,3 +/- 26.8%. SEC was associated to methotrexate (MTX) in 139 pts (24.8%) and was the first line bDMARD in 55 pts (9.8%). The median drug maintenance (MDM) of SEC was 79 weeks (wk) [73-84]. At 52 wk, 245 pts (60%) SpA were still treated with SEC. During the 3-year follow-up, 264 pts discontinued SEC: 180 (68.2%) pts for lack of effectiveness, 47 (17.8%) for adverse events, 14 (5.3%) for others and 23 (8.7%). SEC prescription as first line bDMARD was associated with longer survival versus second line or more: 111 wk [83-138] vs. 69 wk [57-80] (p=0. 017) (
Secukinumab maintenance according to therapeutic line
Secukinumab maintenance in nr-axSpA population
Conclusion: In routine clinical practice, SEC median maintenance was 79 weeks. Fist line administration was the only independent factor associated with improved SEC retention. Lack of effectiveness was the most common reason of discontinuation.
Disclosure of Interests: Benoît Flachaire: None declared, Jean-Guillaume Letarouilly Grant/research support from: Research grant from Pfizer, Céline Labadie: None declared, Nicolas Cohen Speakers bureau: Novartis, Janssen, Vincent Pradel: None declared, Bruno Fautrel Grant/research support from: AbbVie, Lilly, MSD, Pfizer, Consultant of: AbbVie, Biogen, BMS, Boehringer Ingelheim, Celgene, Lilly, Janssen, Medac MSD France, Nordic Pharma, Novartis, Pfizer, Roche, Sanofi Aventis, SOBI and UCB, Guy Baudens: None declared, Pascal Claudepierre Speakers bureau: Janssen, Novartis, Lilly, Corinne Miceli Richard: None declared, Philippe Dieudé: None declared, Jean-Hugues Salmon Speakers bureau: Novartis, Janssen, Jérémie SELLAM: None declared, Eric Houvenagel Speakers bureau: Janssen, Novartis, Marie-Hélène Guyot: None declared, Chi Duc Nguyen: None declared, Xavier Deprez Speakers bureau: Novartis, Janssen, Isabelle CHARY VALCKENAERE: None declared, Pierre Lafforgue Speakers bureau: Novartis, Janssen, Damien LOEUILLE: None declared, Christophe Richez Consultant of: Abbvie, Amgen, Mylan, Pfizer, Sandoz and UCB., Rene-Marc Flipo Speakers bureau: Novartis, Janssen, Lilly, Thao Pham Speakers bureau: Novartis, Janssen, Lilly