

Background: Maintenance of clinical response over time has been shown in individual patients (pts) with polyarticular-course juvenile idiopathic arthritis (pJIA) treated with SC abatacept (ABA). 1 It is unknown whether each individual pt with sustained efficacy also consistently maintains the previously reported shorter-term benefits on patient-reported outcomes (PROs) 2,3 over time.
Objectives: Investigate whether combined efficacy and stringent, optimal PRO responses to ABA treatment are maintained by individual pts with pJIA over time.
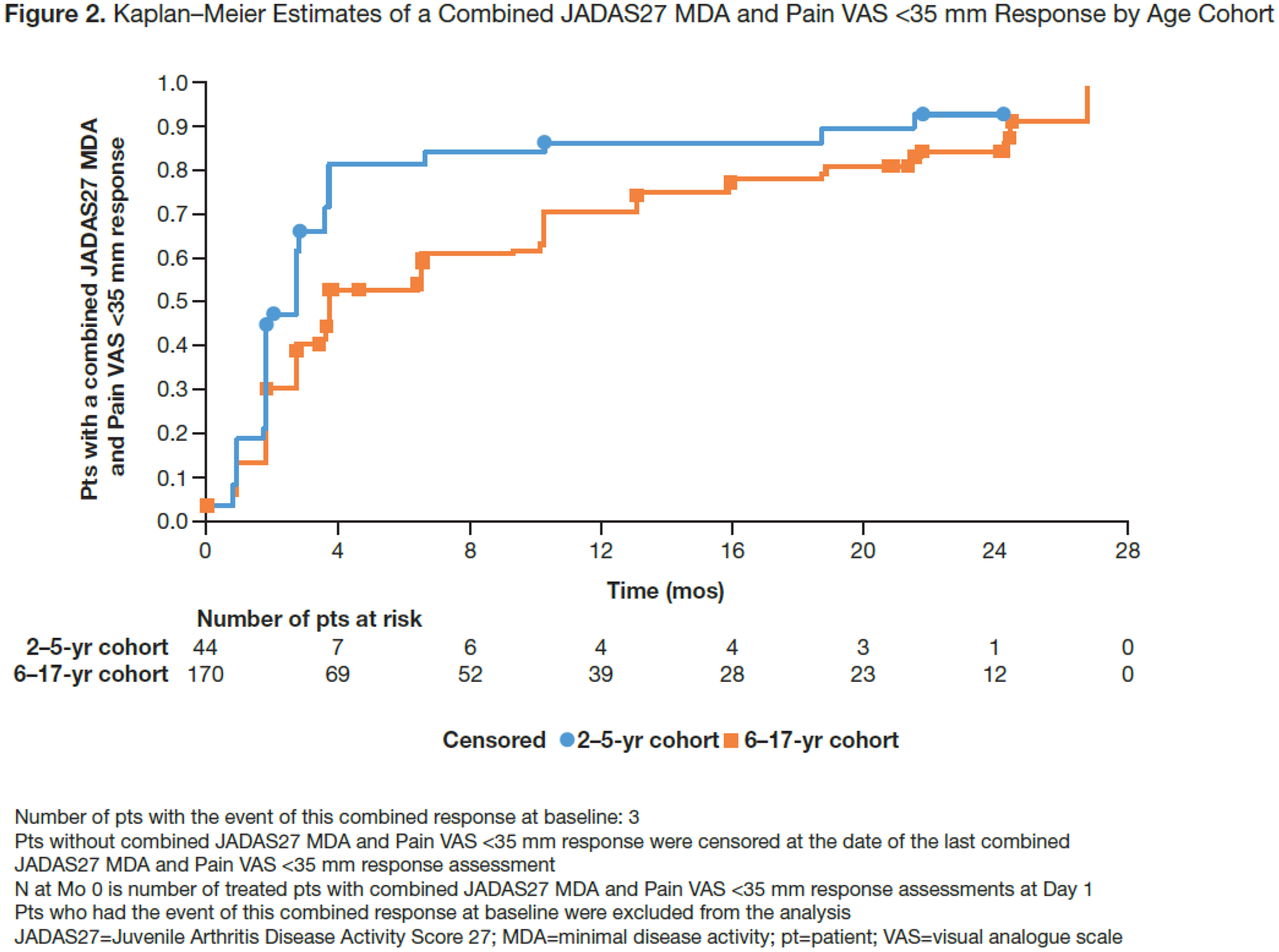
Methods: In this analysis of the intent-to-treat population, pts in two age cohorts (2–5 and 6–17 yrs) who achieved clinical response to weekly SC ABA (10–<25 kg [50 mg], 25–<50 kg [87.5 mg], ≥50 kg [125 mg]) at Mo 4 (time point of primary pharmacokinetic endpoint 4 ) were followed for 2 yrs. Stringent efficacy outcomes (Juvenile Arthritis Disease Activity Score 27 [JADAS27] minimal disease activity [MDA; ≤3.8] and inactive disease [ID; ≤1] status) were combined with optimal PRO endpoints (childhood [C]HAQ-DI=0, Parental Global Assessment [PaGA] ≤1 and Pain visual analogue scale [VAS] <35). Combined efficacy and PRO responses were analysed at Mos 4, 13 and 21.
Results: 219 pts entered the study (46 [21.0%] 2–5 yrs; 173 [79.0%] 6–17 yrs); a subgroup of these pts achieved a clinical response at Mo 4 (
Proportion of pts with combined efficacy and optimal PRO responses at Mos 4, 13 and 21
| Endpoint | Responders at Mo 4 | Responders at Mos 4 and 13* | Responders at Mos 4, 13 and 21* | |||
|---|---|---|---|---|---|---|
| 2–5 yrs (n=46 ) | 6–17 yrs (n=173 ) | 2–5 yrs | 6–17 yrs | 2–5 yrs | 6–17 yrs | |
| JADAS27 MDA and CHAQ-DI=0 | 9 (20) | 34 (20) | 5/9 (56) | 25/34 (74) | 3/9 (33) | 16/34 (47) |
| JADAS27 MDA and PaGA ≤1 | 8 (17) | 14 (8) | 8/8 (100) | 7/14 (50) | 7/8 (88) | 5/14 (36) |
| JADAS27 MDA and Pain VAS <35 mm | 28 (61) | 70 (41) | 25/28 (89) | 58/70 (83) | 21/28 (75) | 43/70 (61) |
| JADAS27 ID and CHAQ-DI=0 | 7 (15) | 20 (12) | 2/7 (29) | 13/20 (65) | 1/7 (14) | 9/20 (45) |
| JADAS27 ID and PaGA ≤1 | 6 (13) | 10 (6) | 4/6 (67) | 4/10 (40) | 4/6 (67) | 4/10 (40) |
| JADAS27 ID and Pain VAS <35 mm | 17 (37) | 31 (18) | 10/17 (59) | 22/31 (71) | 8/17 (47) | 17/31 (55) |
Data are n (%) or n/N (%). *% based on n of pts who achieved response at Mo 4 (denominator)
Kaplan–Meier estimates for median (95% CI) times (mos) to achieving combined efficacy and optimal PRO responses
| Endpoint | 2–5 yrs | 6–17 yrs |
|---|---|---|
| JADAS27 MDA and CHAQ-DI=0 | 21.5 (6.8, NE) | 21.5 (13.1, 24.4) |
| JADAS27 MDA and PaGA ≤1 | NE (15.9, NE) | 24.6 (24.3, NE) |
| JADAS27 MDA and Pain VAS <35 mm | 2.8 (1.9, 2.9) | 3.8 (3.7, 6.6) |
| JADAS27 ID and CHAQ-DI=0 | NE (18.4, NE) | 24.4 (18.7, NE) |
| JADAS27 ID and PaGA ≤1 | NE (21.3, NE) | 24.6 (24.3, NE) |
| JADAS27 ID and Pain VAS <35 mm | 3.8 (3.8, 10.3) | 13.2 (10.3, 15.9) |
NE=not estimable
Conclusion: Many individuals with pJIA who achieved stringent efficacy and PRO measures with weekly SC abatacept by Mo 4 sustained them over 2 years. Time to achieve combined efficacy and Pain VAS <35 response was shorter than that for PaGA ≤1 and CHAQ-DI=0.
REFERENCES:
[1]Ruperto N, et al. Ann Rheum Dis 2019;78:99–100 (abstr OP0056)
[2]Brunner H, et al. Arthritis Rheumatol 2019;71(suppl 10):abstr 2707
[3]Ruperto N, et al. Ann Rheum Dis 2017;76:75 (abstr OP0058)
[4]Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–54

Acknowledgments: Katerina Kumpan, PhD, Caudex; funding: Bristol-Myers Squibb
Disclosure of Interests : Hermine Brunner Consultant of: Hoffman-La Roche, Novartis, Pfizer, Sanofi Aventis, Merck Serono, AbbVie, Amgen, Alter, AstraZeneca, Baxalta Biosimilars, Biogen Idec, Boehringer, Bristol-Myers Squibb, Celgene, EMD Serono, Janssen, MedImmune, Novartis, Pfizer, and UCB Biosciences, Speakers bureau: GSK, Roche, and Novartis, Nikolay Tzaribachev: None declared, Ingrid Louw Consultant of: Amgen, Novartis, Pfizer, Roche (advisory boards), Inmaculada Calvo Grant/research support from: Bristol-Myers Squibb, Clementia, GlaxoSmithKline, Hoffman-La Roche, Merck Sharpe & Dohme, Novartis, Pfizer, Sanofi, Speakers bureau: AbbVie, GlaxoSmithKline, Hoffman-La Roche, Novartis, Francisco Zapata: None declared, Gerd Horneff Grant/research support from: AbbVie, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Speakers bureau: AbbVie, Bayer, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Ivan Foeldvari Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Chugai, Eli Lilly, Novartis, Pfizer, Daniel Kingsbury: None declared, Maria Gastanaga Grant/research support from: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, Roche, Speakers bureau: AbbVie, Roche, Carine Wouters Grant/research support from: GlaxoSmithKline, Pfizer, Roche, Johannes Breedt: None declared, Robert Wong Shareholder of: Bristol-Myers Squibb, Employee of: Bristol-Myers Squibb, Marleen Nys Shareholder of: Bristol-Myers Squibb, Employee of: Bristol-Myers Squibb, Margarita Askelson Consultant of: Bristol-Myers Squibb, Joe Zhuo Shareholder of: Bristol-Myers Squibb, Employee of: Bristol-Myers Squibb, Alberto Martini Consultant of: AbbVie, Eli Lily, EMD Serono, Janssen, Novartis, Pfizer, UCB, Daniel J Lovell Consultant of: Abbott (consulting and PI), AbbVie (PI), Amgen (consultant and DSMC Chairperson), AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb (PI), Celgene, Forest Research (DSMB Chairman), GlaxoSmithKline, Hoffman-La Roche, Janssen (co-PI), Novartis (consultant and PI), Pfizer (consultant and PI), Roche (PI), Takeda, UBC (consultant and PI), Wyeth, Employee of: Cincinnati Children’s Hospital Medical Center, Speakers bureau: Wyeth, Nicolino Ruperto Grant/research support from: Bristol-Myers Squibb, Eli Lily, F Hoffmann-La Roche, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sobi (paid to institution), Consultant of: Ablynx, AbbVie, AstraZeneca-Medimmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lily, EMD Serono, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, Sobi, Takeda, Speakers bureau: Ablynx, AbbVie, AstraZeneca-Medimmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lily, EMD Serono, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Merck, Novartis, Pfizer, R-Pharma, Sanofi, Servier, Sinergie, Sobi, Takeda