

Background: The Clinical Disease Activity Index (CDAI) and the Routine Assessment of Patient Index Data 3 (RAPID3) ascertain rheumatoid arthritis (RA) disease activity and inform treatment decisions. The CDAI has provider and patient components, whilst the RAPID3 only has patient driven measures. During the COVID-19 pandemic, telemedicine visits relied on RAPID3 as a clinical outcome measure and subsequently was incorporated into all clinical visits in addition to the CDAI. On an ad-hoc basis, discrepancies were noted for the disease activity level generated by these two measures. The purpose of this retrospective study was to formally analyze the relationship between these measures.
Objectives: To determine the concordance of the outcome measures RAPID3 with CDAI in patients with established RA as a quality improvement project.
Methods: This is a retrospective study of 49 patients that fulfilled the American College of Rheumatology 2010 criteria for Rheumatoid Arthritis. IRB approval was obtained. The medical records of patients seen between June to October 2020 at the rheumatology department at UF health were reviewed. Data collected included age, gender, race, number of years with RA, Rheumatoid factor (RF) and anti-citrullinated protein antibody (CCP Ab) positivity, disease modifying treatments, ESR and CRP as well as CDAI and RAPID3 scores as calculated by clinic staff. The charts were reviewed by the authors and RAPID3 scores were verified.
Results: The population ranged from 35- 90 years and duration of RA from 1- 30 years. CCP Ab was present in 75% of patients and RF in 71%. Patients were on DMARDs either monontherapy (29%), dual therapy (60%) or triple therapy (10%). Antirheumatic medications used were plaquenil, methotrexate, leflunomide, etanercept, adalimumab, infliximab, tofacitinib, upadacitinib and rituximab. ESR range was 2-110 and CRP 0.2- 83.1. The CDAI and RAPID3 concordance was found to be 37% with RAPID3 being higher in 45% of patients. RAPID3 was lower only in 14% of patients. There was incorrect calculation of the RAPID3 26% of the time by clinic staff.
Patient Population
| Age | 35-90 years |
| Race | African American-13
|
| RF Positive | 35/49 (71)% |
| CCP Ab | 37/49 (75%) |
| Both RF and CCP Ab Positive | 32/49 (65%) |
| Patients on monotherapy | 14/48 (29%) |
| Patients on dual therapy | 29/48 (60%) |
| Patients on triple therapy | 5/48 (10) |
| Antirheumatic Drugs used | Plaquenil, methotrexate, leflunomide, etanercept, adalimumab, infliximab, tofacitinib, upadacitinib, rituximab |
| CDAI and RAPID3 Concordance | 18/49 (37%) |
| RAPID 3 Higher than CDAI | 22/49 (45%) |
| RAPID 3 lower than CDAI | 7/49 (14%) |
| Incorrect Calculation of RAPID3 by clinic staff | 11/42 (26%) |
Scatterplots of three RAPID3 strata. Red dots represent discordant subjects when compared to CDAI. Note: Panel C demonstrates the subjects that were in low disease activity in red that had a high severity RAPID3 score
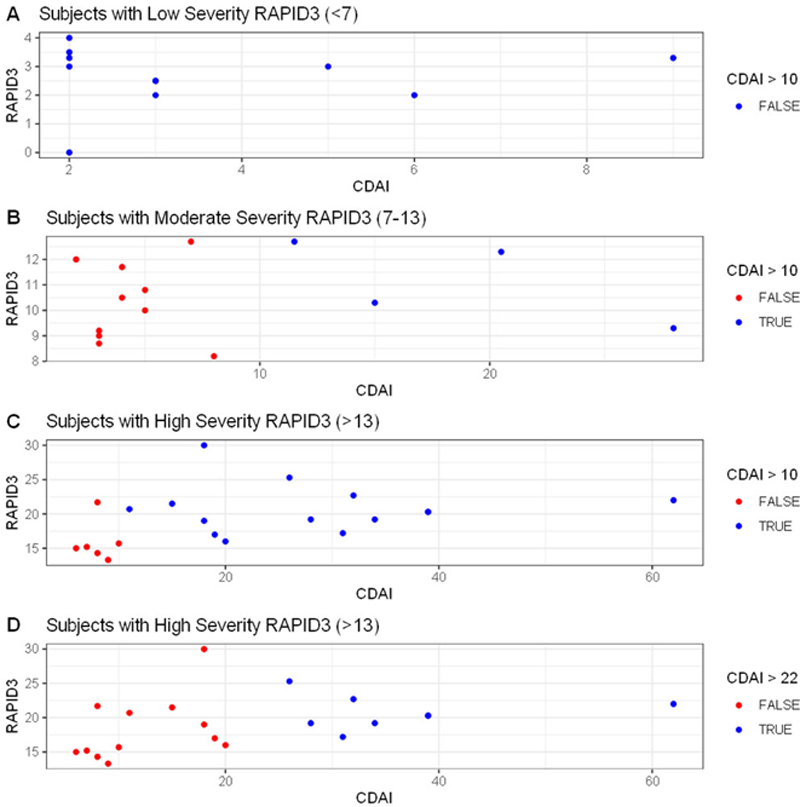
Conclusion: This study shows that RAPID3 may overestimate disease activity level for patients above low disease activity. Treatment escalation based on RAPID3 in discordant patients may be inappropriate. When making treatment decisions, a measure that includes objective physical examination and provider judgment is desirable.
REFERENCES:
[1]Kumar, B. S., Suneetha, P., Mohan, A., Kumar, D. P. & Sarma, K. V. S. Comparison of Disease Activity Score in 28 joints with ESR (DAS28), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire Disability Index (HAQ-DI) & Routine Assessment of Patient Index Data with 3 measures (RAPID3) for assessing disease activity in patients with rheumatoid arthritis at initial presentation. Indian J Med Res 146 , S57–S62 (2017).
[2]Pincus, T., Swearingen, C. J., Bergman, M. & Yazici, Y. RAPID3 (Routine Assessment of Patient Index Data 3), a Rheumatoid Arthritis Index Without Formal Joint Counts for Routine Care: Proposed Severity Categories Compared to Disease Activity Score and Clinical Disease Activity Index Categories. The Journal of Rheumatology 35 , 2136–2147 (2008).
Disclosure of Interests: None declared