

Background: Therapeutic equivalence of CT-P17 to reference adalimumab (ref-adalimumab) has been shown in patients with moderate-to-severe active rheumatoid arthritis (RA) through primary 24-week results [1]. Here, efficacy, pharmacokinetics (PK), safety and immunogenicity results up to 52-week, including transition data from ref-adalimumab to CT-P17 are presented.
Objectives: To evaluate efficacy, PK, safety and immunogenicity when switched from ref-adalimumab to CT-P17 compared to maintaining CT-P17 or ref-adalimumab.
Methods: In this study, 648 moderate-to-severe active RA patients despite methotrexate treatment were randomized (1:1) to either CT-P17 or ref-adalimumab and treated with doses of 40 mg every 2 weeks up to Week 24. Prior to dosing at Week 26, 608 patients were randomized again to either maintaining their treatments or being switched from ref-adalimumab to CT-P17. Efficacy, PK, safety, and immunogenicity were assessed up to Week 52.
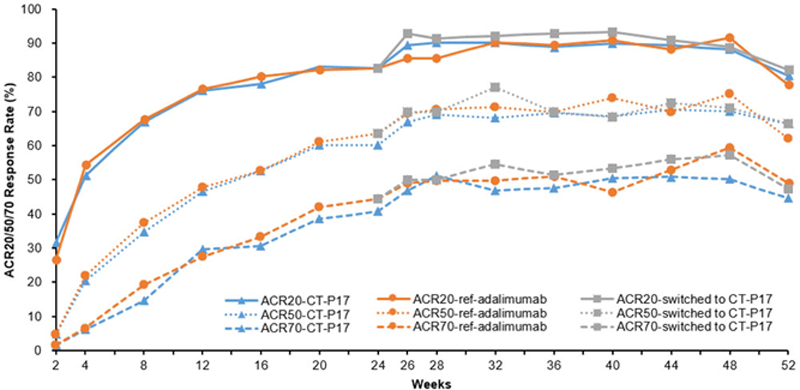
Results: After the second randomization, 303 patients continued with CT-P17, 153 patients continued with ref-adalimumab and 151 patients switched from ref-adalimumab to CT-P17 treatments, up to Week 48. Demographics and baseline characteristics were similar among the 3 groups. Sustained and comparable efficacy in terms of ACR20/50/70 response rates was achieved not only in the maintenance groups (CT-P17 or ref-adalimumab) but also in the switched from ref-adalimumab to CT-P17 group up to Week 52 (
ACR 20/50/70 Response Rates up to 1 YearAbbreviation: ref-adalimumab, reference adalimumab.Note. There were patients who could not visit the study site due to COVID-19 pandemic and were counted as nonresponder for ACR response at Week 52.
In terms of PK, mean trough serum concentration (Ctrough) were maintained after Week 24 in all 3 groups. The observed mean Ctrough were within the reported therapeutic ranges of ref-adalimumab trough levels in RA patients (5-8 μg/mL).
The safety profile after transition was comparable among the 3 groups (
Conclusion: Single transition from ref-adalimumab to CT-P17 was efficacious and safe without increase in immunogenicity. Also, efficacy, PK, safety and immunogenicity profiles were comparable between CT-P17 and ref-adalimumab up to Week 52.
REFERENCES:
[1]J Kay et al, 2020. Poster Presented at ACR Convergence 2020.
Overview of TEAEs from Weeks 26 to 52 (Safety Population – second random subset)
| Patients, n (% ) | Second Randomization | ||
|
CT-P17 Maintenance
|
Ref-ada Maintenance
| Switched to CT-P17 (N=152 ) | |
| ≥1 TEAE | 121 (39.9) | 69 (45.4) | 73 (48.0) |
| ≥1 TESAE | 6 (2.0) | 3 (2.0) | 5 (3.3) |
| ≥1 TEAE leading to study drug discontinuation | 3 (1.0) | 2 (1.3) | 5 (3.3) |
| ≥1 TEAE classified as hypersensitivity/allergic reactions | 2 (0.7) | 1 (0.7) | 0 (0) |
| ≥1 TEAE classified as injection site reactions | 1 (0.3) | 4 (2.6) | 1 (0.7) |
| ≥1 TEAE classified as infection | 54 (17.8) | 41 (27.0) | 28 (18.4) |
| ≥1 TEAE classified as malignancy | 0 (0) | 1 (0.7) | 0 (0) |
Abbreviations: Ref-ada, reference adalimumab; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
Disclosure of Interests: Daniel Furst Speakers bureau: CME, Consultant of: Amgen, Corbus, Galapagos, Horizon, Kadmon, Pfizer, Talaris, Grant/research support from: Corbus, CSL Behring, Galapagos, Gilead, GSK, Horizon, Kadmon, Novartis, Pfizer, Roche/Genetech, Talaris, Edward Keystone Speakers bureau: Amgen, AbbVie, F. Hoffmann-La Roche Inc., Janssen Inc., Merck, Novartis, Pfizer Pharmaceuticals, Sanofi Genzyme, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb Company, Celltrion Inc., Myriad Autoimmune, F. Hoffmann-La Roche Inc, Gilead, Janssen Inc., Lilly Pharmaceuticals, Merck, Pfizer Pharmaceuticals, Sandoz, Sanofi-Genzyme, Samsung Bioepis, Grant/research support from: Amgen, Merck, Pfizer Pharmaceuticals, PuraPharm, Jonathan Kay Consultant of: AbbVie, Inc., Boehringer Ingelheim GmbH, Celltrion Healthcare Co. Ltd., Jubilant Radiopharma, Merck & Co., Inc., Pfizer Inc., Samsung Bioepis, Sandoz Inc., Scipher Medicine, UCB, Inc., Grant/research support from: Paid to the University of Massachusetts Medical School: Gilead Sciences Inc., Novartis Pharmaceuticals Corp., Pfizer Inc., Janusz Jaworski: None declared, Rafal Wojciechowski: None declared, Piotr Wiland Speakers bureau: Eli Lilly, Sanofi Aventis, Novartis, Sandoz, Consultant of: Eli Lilly, Novartis, Sandoz, Anna Dudek: None declared, Marek Krogulec: None declared, Sławomir Jeka Speakers bureau: Novartis, Pfizer, Roche, Lilly, Teva, MSD, Abbvie, Sandoz, Egis, Medac, Consultant of: Novartis, Pfizer, Roche, Lilly, Teva, MSD, Abbvie, Sandoz, Egis, Medac, Agnieszka Zielinska: None declared, Jakub Trefler: None declared, Katarzyna Bartnicka-Masłowska: None declared, Magdalena Krajewska-Wlodarczyk Speakers bureau: Abbvie, Eli Lilly, Novartis, Roche, Piotr Klimiuk: None declared, Sang Joon Lee Employee of: Celltrion, Inc., Sung Hyun Kim Employee of: Celltrion, Inc., YunJu Bae Employee of: Celltrion, Inc., GoEun Yang Employee of: Celltrion, Inc., JaeKyoung Yoo Employee of: Celltrion, Inc., TaeKyung Kim Employee of: Celltrion, Inc.