

Background: Adalimumab (ADL) is typically self-administered every 2 weeks (W) as a subcutaneous (s.c.) injection by patients (pts) for diverse indications, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and psoriasis (PsO). Conflicting evaluations of local tolerance to formulations containing citrate buffer have created insecurities among health care professionals and pts. 1,2
Objectives: To evaluate local tolerance of SDZ-ADL (GP2017), a biosimilar ADL with low citrate concentration (1.2 mM), 3 in 466 healthy volunteers (HV) and 408 pts (RA: 177 and PsO: 231 including PsA: 52) from four phase I pharmacokinetic (PK) and two phase III confirmatory studies.
Methods: HV evaluated their injection site pain (ISP) using a Visual Analogue Scale (VAS) of 0–100 mm. HV received a single 40 mg/0.8 mL s.c. injection and pts received SDZ-ADL every 2W during 48–51W duration of study. Injection site reactions (ISR) as well as adverse events (AEs) were assessed by investigators during the clinical studies. Details of study designs have been reported previously. 4–7
Results: Overall, 456 (97.9%) HV experienced no ISR. Ten HV experienced ISR. These were mostly of mild intensity; only 1 (0.2%) had an ISR of moderate intensity. 96.6% of HV experienced no pain (VAS score 0–4 mm)
8
at 1-hour post-dose (
Conclusion: The proportion of HV and pts experiencing ISR and ISP after administration of SDZ-ADL was low, with no events leading to treatment or study discontinuation. These results call into question the clinical impact of citrate and its concentration in ADL formulations on the incidence and intensity of ISP.
REFERENCES:
[1]Nash et al. Rheumatol Ther . 2016;3:257–70.
[2]NHS. Regional medicines optimisation committee briefing, best value biologicals: adalimumab update 6. July 2019.
[3]
[4]Blauvelt A, et al. Br J Dermatol . 2018;179:623–31.
[5]Wiland P, et al. BioDrugs 2020;34:809–23.
[6]Richter OV, et al. Expert Opin Biol Ther . 2019;19:1057–64.
[7]Richter OV, et al. Expert Opin Biol Ther . 2019;19:1075–83.
[8]Hawker GA, et al. Arthritis Care Res . 2011;63:240–52.
Proportion of HV with ISP in phase I PK studies
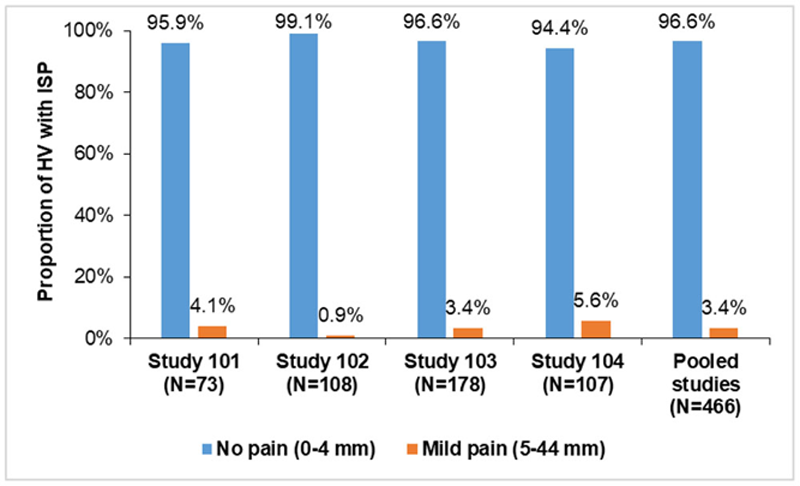
ISP and ISR results from phase I PK and phase III confirmatory studies
| Phase I PK studies | |||||
| Study |
101
|
102
|
103
|
104
| Pooled studies (N=466 ) |
| VAS scores (mm) at 1-hour post-dose | |||||
| Mean (SD) | 0.89 (2.07) | 0.07 (0.52) | 1.03 (1.71) | 1.03 (2.49) | 0.79 (1.84) |
| Median | 0 | 0 | 1 | 0 | 0 |
| ISR scores at 1-hour post-dose, n (% ) | |||||
| None | 73 (100) | 106 (98.2) | 178 (100) | 99 (92.5) | 456 (97.9) |
| Mild | 0 (0) | 1 (0.9) | 0 (0) | 8 (7.5) | 9 (1.9) |
| Moderate | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 1 (0.2) |
| Phase III confirmatory studies | |||||
| ADACCESS 5 * (PsO and PsA pts ) | ADMYRA 6 * (RA pts ) | ||||
| W0–17 (N=231) | W0–51 (N=168; including pts re-randomised to continue SDZ-ADL after W17) | W0–24 (N=177) | W0–48 (N=177; all pts continued SDZ-ADL after W24) | ||
| Dosage | Induction 80 mg W0, then 40 mg EoW s.c. | 40 mg EoW s.c. | 40 mg EoW s.c. | 40 mg EoW s.c. | |
| Study duration, W | 17 | 51 | 24 | 48 | |
| AEs - ISR, n (%), events | 15 (6.5), 34 | 9 (5.4), 26 | 7 (4.0), 11 | 7 (4.0), 12 | |
| Mild | 14 (6.1), 30 | 9 (5.4), 26 | 7 (4.0), 11 | 7 (4.0), 12 | |
| Moderate | 1 (0.4), 4 | 0 | 0 | 0 | |
| AEs - ISP (reported as ISR), n (% ) | 3 (1.3) | 1 (0.6) | 2 (1.1) | 2 (1.1) | |
*ADACCESS and ADMYRA were switch studies, therefore, only pts exposed to SDZ-ADL throughout the study period are included here. EoW, every other week, N, number of HV or pts
Disclosure of Interests: Piotr Wiland Speakers bureau: Celltrion, Celgene, Eli Lilly, Novartis, Pfizer, Sandoz, Sanofi-Aventis, Consultant of: Celltrion, Celgene, Eli Lilly, Novartis, Pfizer, Sandoz, Sanofi-Aventis, Andrew Blauvelt Speakers bureau: AbbVie, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Leo, Novartis, Pfizer, Rapt, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma, and UCB Pharma., Consultant of: AbbVie, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Evommune, Forte, Galderma, Incyte, Janssen, Leo, Novartis, Pfizer, Rapt, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma, and UCB Pharma., Lena Lemke Employee of: Hexal AG, Oliver von Richter Employee of: Hexal AG, Alison Balfour Employee of: Hexal AG, Fabricio Furlan Employee of: Hexal AG, Norman Gaylis: None declared