

Background: Choosing the best treatment strategy for a patient is one of the most difficult issues in modern rheumatology, as there are various factors affecting drug therapy in chronic diseases, such as efficacy, safety, and compliance. Physicians take care of long-term retention rate and responses for discontinuation of candidate drug.
Objectives: The purpose of this study to assess the drug survival of certolizumab pegol (CZP) in patients with axial spondyloarthritis (ax-SpA) and to identify the predictors and reasons for discontinuation.
Methods: Data on patient characteristics, demographics, diagnosis, duration of disease, treatment and outcomes have been collected since 2011 in Turkish Biologic (TURKBIO) Registry. By the end of December 2020, 410 ax-SpA patients received CZP and were included. Kaplan Meier plot was used for drug survival analysis. Cox regression analysis was performed to evaluate the predictor associated with drug survival.
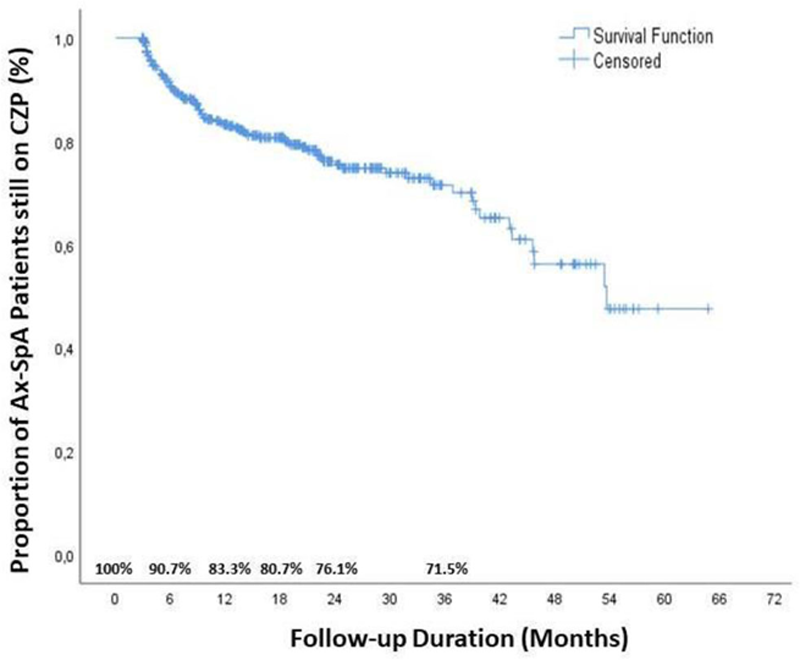
Results: During the median 54 months follow-up, 92 (22.4%) patients discontinued the CZP treatment. The reasons for discontinuation: ineffectivity was 58.7% (n=54), adverse events was 6.5%, pregnancy was 3.3% and surgery was 4.3%. The baseline characteristics of patients continue with CZP and discontinuation due to ineffectiveness were shown in the
Conclusion: Real life experience from this nationwide TURKBIO registry show higher long-term retention rate of CZP in ax-SpA. Higher baseline disease activity and functional limitation predict discontinuation of CZP. Adding NSAIDs and csDMARDs to the treatment of the patient with poor prognosis cannot increase retention rates.
Drug survival of CZP in patients with Ax-SpA
Baseline characteristics of ax-SpA patients who continue and discontinue CZP
| All patients (n=410 ) | Continue to CZP (n=318 ) | Discontinue to CZP* (n=54 ) | p | ||
| Females, n (% ) | 185 (49,7) | 157 (49,4) | 28 (51,9) | 0,736 | |
| Age, years | 42 (34-49) | 41 (34-49) | 45 (34-54) | 0,064 | |
| Symptom duration, years | 11 (7-17) | 11 (6-16) | 12 (8,5-20) | 0,054 | |
| HLA-B27, n (% ) | 150 (63,8) | 129 (64,5) | 21 (60) | 0,609 | |
| Previous bDMARDs, n (% ) | Adalimumab | 54 (14,5) | 42 (13,2) | 12 (22,2) | 0,082 |
| Etanercept | 53 (14,2 ) | 40 (12,6 ) | 13 (24,1 ) | 0,025 | |
| Golimumab | 11 (3) | 7 (2,2) | 4 (7,4) | 0,060 | |
| Infliximab | 39 (10,5) | 35 (11) | 4 (7,4) | 0,425 | |
| Co-treated drugs, n (% ) | NSAID | 206 (55,4 ) | 169 (53,1 ) | 37 (68,5 ) | 0,036 |
| Methotrexate | 35 (9,4 ) | 22 (6,9 ) | 13 (24,1 ) | <0,001 | |
| Sulphasalazine | 61 (16,4 ) | 40 (12,6 ) | 21 (38,9 ) | <0,001 | |
| Leflunomide | 5 (1,3 ) | 2 (0,6 ) | 3 (5,6 ) | 0,023 | |
| ESH, mm/h | 21,5 (10-37) | 21 (10-37) | 23 (10-34) | 0,999 | |
| CRP, mg/dl | 7 (3-20) | 7 (3-20) | 7 (3-22) | 0,727 | |
| HAQ | 0,63 (0,25-0,94 ) | 0,5 (0,25-0,88 ) | 0,75 (0,38-1,25 ) | 0,009 | |
| BASFI | 21 (7-45 ) | 20,5 (6-41 ) | 31 (13-58 ) | 0,011 | |
| BASDAI | 30,5 (13-52 ) | 30 (12-50 ) | 43 (23-61,5 ) | 0,002 | |
| ASDAS | 2,7 (1,8-3,7) | 2,7 (1,8-3,6) | 2,9 (2,3-4) | 0,062 | |
*Discontinue due to ineffectivity.
REFERENCES:
[1]Iannone F, et al. Effectiveness of Certolizumab-Pegol in Rheumatoid Arthritis, Spondyloarthritis, and Psoriatic Arthritis Based on the BIOPURE Registry: Can Early Response Predict Late Outcomes? Clin Drug Investig. 2019;39(6):565-575.
Disclosure of Interests: None declared.