

Background: Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA). It was approved in the United States (US) in December 2017 for use in combination with non-biologic disease-modifying antirheumatic drugs (DMARDs).
Objectives: This analysis of real-world data assessed demographic and baseline clinical characteristics, as well as treatment persistence/adherence, in patients (pts) with PsA who had newly initiated tofacitinib treatment.
Methods: This retrospective cohort study included pts aged ≥18 years in the Truven MarketScan™ US Commercial and Medicare Supplemental Claims and Encounters database with ≥1 tofacitinib claim (first = index) between 14 December 2017–30 April 2019, and PsA diagnoses (≥1 inpatient or ≥2 outpatient [30–365 days apart]) on or within 12 months pre-index. Pts were continuously enrolled for 12 months pre-index and 6 months post-index, with no pre-index claims for tofacitinib. Pt demographic and clinical characteristics on the day of index, history of advanced therapy treatment (≥1 claim for biologic DMARDs or apremilast within 12 months pre-index) and tofacitinib treatment regimen (monotherapy or combination therapy [≥1 claim for conventional synthetic DMARDs or apremilast on or within 90 days post-index]) were recorded. Outcomes at 6 months post-index included tofacitinib persistence (<60-day gap without tofacitinib treatment) and adherence (proportion of days covered ≥80% and medication possession ratio [data not shown]). A sensitivity check was performed by analysing a sub-cohort that excluded pts with a diagnosis of rheumatoid arthritis (RA) on or within 12 months pre-index and within 6 months post-index.
Results: Of 17 321 pts receiving tofacitinib, 440 pts met the inclusion criteria for the overall cohort, with 315 pts included in the sub-cohort. In the overall cohort, pts were mostly female, with a mean age of 52.3 years and a mean PsA duration of 738 days (data not shown). Most pts were exposed to ≥1 advanced therapy within 12 months pre-index (mean = 1.1; range = 0–4); most commonly secukinumab (
History of advanced therapy on or within 12 months pre-index in pts with PsA
|
All pts
|
Pts with no RA
a
|
|
| Pts exposed to advanced therapy, n (%) b | ||
| Any advanced therapy c | 336 (76.4) | 241 (76.5) |
| Secukinumab | 113 (25.7) | 91 (28.9) |
| Adalimumab | 93 (21.1) | 58 (18.4) |
| Apremilast | 81 (18.4) | 59 (18.7) |
| Etanercept | 70 (15.9) | 47 (14.9) |
| Unique advanced therapy prescriptions | ||
| Mean (SD) | 1.1 (0.8) | 1.1 (0.8) |
| Median (IQR) | 1 (1–2) | 1 (1–2) |
| Range | 0–4 | 0–4 |
| Number of unique advanced therapies, n (%) | ||
| 0 | 104 (23.6) | 74 (23.5) |
| 1 | 214 (48.6) | 153 (48.6) |
| 2 | 100 (22.7) | 73 (23.2) |
| ≥3 | 22 (5.0) | 15 (4.8) |
a Excludes pts with a diagnosis of RA on or within 12 months pre-index and within 6 months post-index; b Pts with ≥1 claim for biologic DMARDs or apremilast within 12 months pre-index; c Other advanced therapies reported in <10% of all pts were abatacept, certolizumab, golimumab, infliximab, ixekizumab and ustekinumab
IQR, interquartile range; N, number of pts per cohort; n, number of pts per category; SD, standard deviation
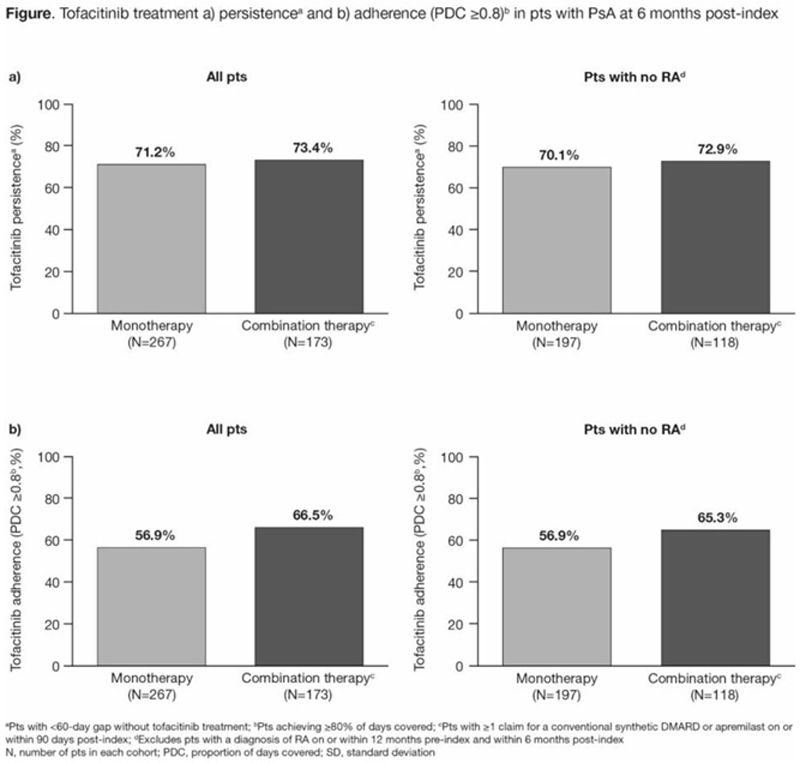
Conclusion: This analysis of US-based claims data indicated that pts newly initiated tofacitinib treatment an average of 2 years after PsA diagnosis, with the majority (>60%) of pts receiving tofacitinib as monotherapy. High levels of persistence and adherence to tofacitinib were observed 6 months after treatment initiation. Findings were similar when pts with PsA who also had a diagnosis of RA were excluded. Data were limited in that claims data cannot confirm that pts took the medication for which they filed a claim.
Acknowledgements: Study sponsored by Pfizer Inc. Medical writing support was provided by Gemma Turner, CMC Connect, and funded by Pfizer Inc.
Disclosure of Interests: Philip J Mease Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Genentech, Janssen, Novartis, Pfizer Inc, UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer Inc, Sun, UCB, Grant/research support from: AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer Inc, Sun, UCB, Pamela Young Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, David C Gruben Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Lara Fallon Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Rebecca Germino Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Arthur Kavanaugh Grant/research support from: Pfizer Inc.