

Background: Guselkumab (GUS), an anti-interleukin-23p19-subunit monoclonal antibody, demonstrated efficacy vs placebo (PBO) in achieving American College of Rheumatology 20% improvement (ACR20) response in patients (pts) with active psoriatic arthritis (PsA) in two phase 3 trials, DISCOVER-1 & 2. 1,2
Objectives: To assess the differential treatment effects of GUS across individual components of ACR response in PsA pts participating in the DISCOVER-1 & 2 trials.
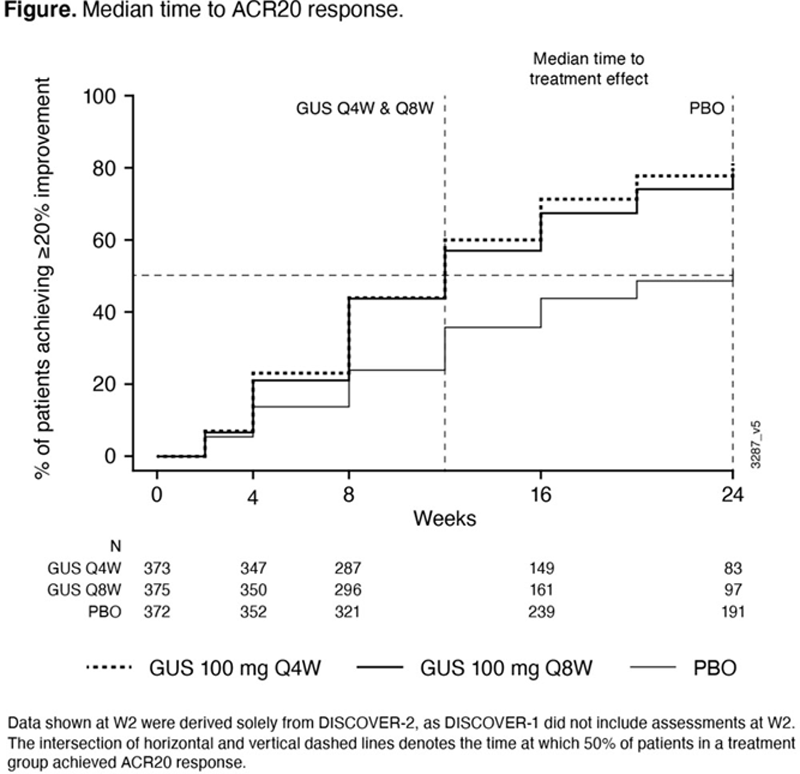
Methods: In DISCOVER-1 & 2, 1120 pts were randomized & treated with GUS 100 mg every 4 weeks (Q4W; N=373); GUS 100 mg at Week (W)0 and W4, then Q8W (N=375); or matching PBO (N=372). Pts were evaluated by independent joint assessors at study visits. ACR20 response is defined as ≥20% improvement from baseline in both tender joint count (0-68 [TJC68]) and swollen joint count (0-66 [SJC66]) and ≥20% improvement from baseline in ≥3 of 5 assessments: Patient Assessment of Pain [Pt Pain], Patient Assessment of Global Disease Activity (arthritis) [PtGA], Physician Assessment of Global Disease Activity [PGA], Patient assessment of physical function as measured by Disability Index of the Health Assessment Questionnaire (HAQ-DI), and C-reactive protein (CRP). For each ACR component, achievement of ≥20% improvement from baseline was assessed over time through W24 for the combined (Q4W+Q8W) GUS groups, and median time to onset of treatment effect was determined with Kaplan-Meier curves by randomized group.
Results: Median time to response for all components except SJC66 occurred earlier with GUS than PBO. Time to onset of ACR20 treatment effect is shown in
Conclusion: GUS demonstrated ACR20 improvements with separation from PBO in ACR components as early as W4, which is consistent with reduced inflammation by GUS and prior serological studies. 3 At early study time points, both pts and physicians were able to discern improvements in signs and symptoms of arthritis that rapidly followed reductions in systemic inflammation (CRP). The predominant drivers of ACR20 response rates at W24 in GUS pts were SJC66/TJC68/PGA.
REFERENCES:
[1]Deodhar A et al. Lancet. 2020;395:1115-25
[2]Mease P et al. Lancet. 2020;395:1126-36
[3]Siebert S et al. EULAR 2020. Presentation OP0229
| W4 | W8 | W12 | W16 | W20 | W24 | |
| ACR20 | 20 | 39 | 50 | 56 | 60 | 61 |
| HAQ-DI score | 36 | 45 | 52 | 54 | 56 | 57 |
| SJC66 | 61 | 74 | 84 | 86 | 87 | 88 |
| TJC68 | 48 | 65 | 75 | 79 | 80 | 81 |
| PGA | 50 | 67 | 74 | 78 | 81 | 81 |
| PtGA | 35 | 48 | 58 | 59 | 62 | 64 |
| Pt Pain | 32 | 48 | 55 | 58 | 61 | 63 |
| CRP | 56 | 60 | 62 | 63 | 64 | 64 |
Disclosure of Interests: Peter Nash Consultant of: AbbVie, Bristol Myers Squibb, Boehringer, Celgene, Gilead, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sandoz, Sun, Grant/research support from: AbbVie, Bristol Myers Squibb, Boehringer, Celgene, Gilead, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Sandoz, Sun, Iain McInnes Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, and UCB, Christopher T. Ritchlin Consultant of: AbbVie, Amgen, Janssen, Eli Lilly, Gilead, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, and UCB, Wen-Chan Tsai Consultant of: AbbVie, Eli Lilly, Janssen, Pfizer, and Novartis, Ying Ying Leung Consultant of: Abbvie, Eli Lilly, Janssen, and Novartis, Lai-Shan Tam Consultant of: AbbVie, Boehringer Ingelheim, Janssen, Lilly, Pfizer, and Sanofi, Grant/research support from: Amgen, Boehringer Ingelheim, Janssen, GSK, Novartis, and Pfizer, Daniel Furtner Employee of: Janssen, a division of Johnson & Johnson Pte. Ltd., May Shawi Employee of: Janssen Research and Development, LLC, Stephen Xu Employee of: Janssen Research and Development LLC, Shihong Sheng Employee of: Janssen Research and Development, LLC, Alexa Kollmeier Employee of: Janssen Research and Development, LLC, Atul Deodhar Speakers bureau: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB.