

Background: In the Phase 3 DISCOVER-1 1 & DISCOVER-2 2 trials, guselkumab (GUS), a human monoclonal antibody targeting the IL-23p19-subunit, was effective in psoriatic arthritis (PsA) across joint & skin endpoints. At Week 24 (W24), GUS benefit was consistent regardless of baseline (BL) demographic & disease characteristics. 3
Objectives: We assessed whether GUS efficacy was sustained through W52 in pooled DISCOVER-1 & -2 patients (pts) across select BL subgroups.
Methods: Adults with active PsA despite standard therapies were enrolled in DISCOVER-1 (swollen [SJC] ≥3 & tender joint count [TJC] ≥3, C-reactive protein [CRP] ≥0.3 mg/dL) & DISCOVER-2 (SJC ≥5 & TJC ≥5, CRP ≥0.6 mg/dL). 31% of DISCOVER-1 pts had received 1-2 prior tumor necrosis factor inhibitors; DISCOVER-2 pts were biologic naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO). Pts randomized to PBO received GUS 100 mg Q4W starting at W24 & were excluded from these analyses assessing maintenance of effect from W24 to W52. GUS effects on joint (American College of Rheumatology [ACR]20/50/70) & skin (Investigator’s Global Assessment [IGA=0/1 + ≥2-grade reduction from W0] in pts with ≥3% body surface area [BSA] with psoriasis & IGA ≥2 at W0) endpoints were evaluated by pt BL SJC, TJC, conventional synthetic disease-modifying antirheumatic drug (csDMARD) use, body mass index (BMI), PsA duration, & % BSA with psoriasis. Missing data were imputed as nonresponse through W52.
Results: BL pt characteristics in DISCOVER-1 (N=381) & DISCOVER-2 (N=739) were well balanced across randomized groups.
1,2
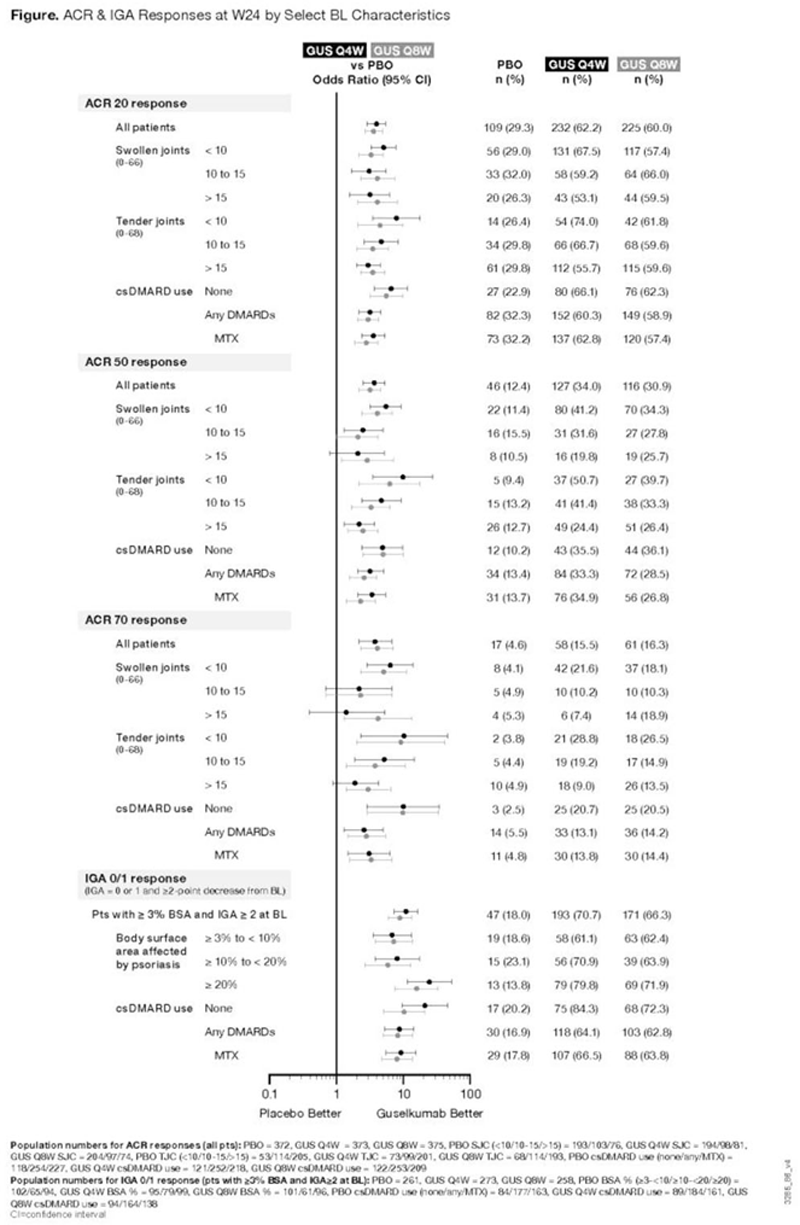
Among 1120 pooled pts, mean SJC was 11, mean TJC was 21, & 68% used csDMARDs (primarily methotrexate [MTX]). At W24, 62% (232/373) & 60% (225/375), respectively, of GUS Q4W- & Q8W-treated pts achieved ACR20 vs 29% (109/372) of PBO, with GUS effect consistently observed across pt BL subgroups (
Conclusion: Treatment with GUS 100 mg Q4W & Q8W resulted in sustained improvement in signs & symptoms of active PsA through W52 regardless of pt BL characteristics.
REFERENCES:
[1]Deodhar A, et al. Lancet 2020;395:1115-25;
[2]Mease P, et al. Lancet 2020;395:1126-36;
[3]Deodhar A, et al. American College of Rheumatology 2020; Poster P0908.
Figure 1
ACR & IGA Responses at Weeks 24 & 52 & by Select BL Characteristics
| Guselkumab Q4W | Guselkumab Q8W | |||
| N=373 | N=375 | |||
| Week 24 | Week 52 | Week 24 | Week 52 | |
| ACR20, % | 62 | 72 | 60 | 70 |
| SJC (<10/10-15/>15) | 68/59/53 | 79/61/67 | 57/66/60 | 68/68/76 |
| TJC (<10/10-15/>15) | 74/67/56 | 73/76/69 | 62/60/60 | 75/68/68 |
| csDMARD use (none/any/MTX) | 66/60/63 | 80/68/68 | 62/59/57 | 73/68/68 |
| ACR50, % | 34 | 49 | 31 | 45 |
| SJC (<10/10-15/>15) | 41/32/20 | 58/39/38 | 34/28/26 | 46/40/49 |
| TJC (<10/10-15/>15) | 51/41/24 | 58/53/43 | 40/33/26 | 52/46/43 |
| csDMARD use (none/any/MTX) | 36/33/35 | 53/46/48 | 36/29/27 | 51/42/40 |
| ACR70, % | 16 | 27 | 16 | 27 |
| SJC (<10/10-15/>15) | 22/10/7 | 32/20/24 | 18/10/19 | 30/23/26 |
| TJC (<10/10-15/>15) | 29/19/9 | 34/32/22 | 27/15/14 | 35/28/24 |
| csDMARD use (none/any/MTX) | 21/13/14 | 30/26/27 | 21/14/14 | 34/24/23 |
| N=273 | N=258 | |||
| IGA 0/1, % | 71 | 80 | 66 | 71 |
| BSA % with psoriasis
| 61/71/80 | 76/87/79 | 62/64/72 | 67/72/74 |
| csDMARD use (none/any/MTX) | 84/64/67 | 87/77/78 | 72/63/64 | 77/68/68 |
Disclosure of Interests: Christopher T. Ritchlin Consultant of: AbbVie, Amgen, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, and UCB Pharma, Grant/research support from: AbbVie, Amgen, and UCB Pharma, Philip J Mease Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN, and UCB Pharma, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, SUN, and UCB Pharma, Wolf-Henning Boehncke Speakers bureau: AbbVie, Almirall, Celgene, Janssen, Leo, Eli Lilly, Novartis, UCB Pharma, Consultant of: AbbVie, Almirall, Celgene, Janssen, Leo, Eli Lilly, Novartis, UCB Pharma, Grant/research support from: Pfizer, John Tesser Speakers bureau: AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Crescendo Biosciences/Myriad, GlaxoSmithKline, Genentech, Janssen, Eli Lilly, and Pfizer, Consultant of: AbbVie, AstraZeneca, Bristol Myers Squibb, Gilead, Janssen, Eli Lilly, Novartis, and Pfizer, Grant/research support from: AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Horizon, Janssen, Eli Lilly, Merck KG, Novartis, Pfizer, Sandoz, Sun Pharma, Setpoint, and UCB Pharma, Elena Schiopu Consultant of: Janssen, Grant/research support from: Janssen, Soumya D Chakravarty Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Scientific Affairs, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Research & Development, LLC, Elizabeth C Hsia Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Research & Development, LLC, May Shawi Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Global Services, LLC, Yusang Jiang Employee of: Cytel, Inc., providing statistical support (funded by Janssen), Shihong Sheng Shareholder of: Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary, Employee of: Janssen Research & Development, LLC, Joseph F. Merola Consultant of: AbbVie, Arena, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB Pharma, Iain McInnes Consultant of: AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB Pharma, Grant/research support from: Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, and UCB Pharma, Atul Deodhar Speakers bureau: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB Pharma, Grant/research support from: AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, UCB Pharma.