

Background: Inflammatory back pain (IBP) is a common symptom of axial disease in patients with psoriatic arthritis (PsA). The reported prevalence of axial disease in patients with PsA is quite variable and must be taken into account while choosing treatment strategy. Netakimab (NTK) is an anti-interleukin-17A monoclonal antibody approved for psoriasis, ankylosing spondylitis, PsA in Russia and Belarus.
Objectives: A subanalysis was aimed to investigate the ACR (American College of Rheumatology) 20/50/70 response rate in PsA patients with/without the axial disease, defined by the presence of IBP according to self-reported ASAS IBP criteria, 2009 at baseline.
Methods: PATERA is an ongoing phase 3 international double-blind, placebo-controlled clinical study (NCT03598751). 194 adult patients with PsA (CASPAR criteria, 2006) with inadequate response to csDMARD or one TNFi, were randomly assigned to receive NTK 120mg or placebo at weeks 0,1,2,4,6,8,10,14,18,22. The ACR response was calculated in NTK-treated patients with IBP (IBP(+)) and NTK-treated patients without IBP (IBP(-)) according to self-reported ASAS IBP criteria, 2009. Patients with missing values for categorical variables were considered as non-responders in the analysis.
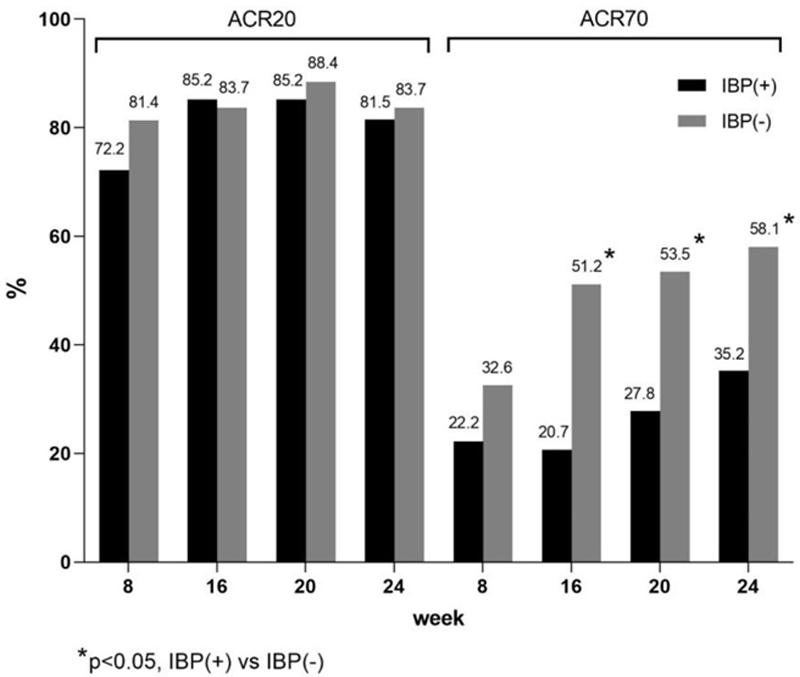
Results: 97 PsA patients (N=54 IBP(+), N=43 IBP(-)) received NTK. Both subpopulations were comparable in gender, age, and PsA activity at baseline. There were no significant differences in ACR20 achievement between the groups (
Conclusion: NTK is effective in PsA treatment irrespectively of the presence of axial disease. Both IBP(-) and IBP(+) subpopulations achieved ACR20/50/70 as well, however, the benefit in IBP(-) patients was more pronounced.
ACR response rates
Acknowledgements: This study was sponsored by JSC BIOCAD.
Disclosure of Interests: Tatiana Korotaeva Speakers bureau: Abbvie, Biocad, Eli Lilly, Johnson & Johnson, Janssen, Novartis, Pfizer, UCB, Inna Gaydukova Speakers bureau: Abbvie, Biocad, Eli Lilly, MSD, Novartis, Pfizer, Sandoz, V Mazurov: None declared., Aleksey Samtsov: None declared., Vladislav Khayrutdinov: None declared., Andrey Bakulev: None declared., Alena Kundzer: None declared., Nikolaj Soroka: None declared., Anna Eremeeva Employee of: Biocad.