

Background: Several factors such as effectiveness, safety and compliance affect the drug survival in chronic disorders. Physicians take care of long-term retention rate and responses for discontinuation of candidate drug. Identification of predictors of clinical response to certolizumab-pegol (CZP) may aid the decision-making process for treating patients psoriatic arthritis (PsA).
Objectives: The purpose of this study to assess the drug survival of certolizumab pegol (CZP) in patients with PsA and to identify the predictors and reasons for discontinuation.
Methods: Data on patient characteristics, demographics, diagnosis, disease duration, treatment and outcomes have been collected since 2011 in Turkish Biologic (TURKBIO) Registry. By the end of December 2020, 68 PsA patients received CZP and were included. Kaplan Meier plot was used for drug survival analysis. Cox regression analysis was performed to evaluate the predictors associated with drug survival.
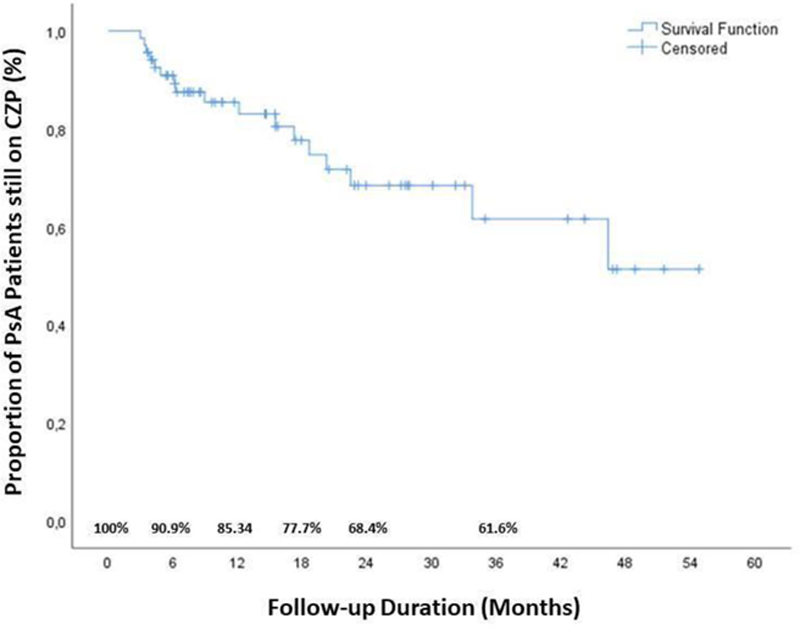
Results: During the median 47 months follow-up, 17 patients discontinued the CZP treatment. The reasons for discontinuation were ineffectivity (35.3%), adverse event (17.6%) and pregnancy (5.9%). The baseline characteristics of the patients who continued and discontinued CZP were shown in the
Conclusion: Real life experience from this nationwide TURKBIO registry show that the retention rate of CZP in PsA are lower in older patients and with longer disease duration. Moreover, bDMARD naive patients have higher retention rate.
Drug survival of CZP in patients with PsA
Baseline characteristics of PsA patients who continue and discontinue CZP
| All Patients (n=68 ) | Continue to CZP (n=51 ) | Discontinue to CZP (n=17 ) | p | |
| Females, n (% ) | 52 (76,5) | 37 (72,5) | 15 (88,2) | 0,322 |
| Age, years | 44 (36-57 ) | 40 (35-53 ) | 51 (42-60 ) | 0,012 |
| Disease Duration, years | 9 (5-13 ) | 8 (5-12 ) | 14 (10,5-17 ) | 0,002 |
| Symptom duration, years | 11 (7-16 ) | 10 (7-15 ) | 15,5 (11,5-20 ) | 0,014 |
| Order of CZP in bDMARDs | 1 (1-2) | 1 (1-2) | 2 (1-2) | 0,062 |
| HLA-B27, n (% ) | 9 (28,1 ) | 3 (14,3 ) | 6 (54,5 ) | 0,035 |
| ESR, mm/h | 23,5 (11-37) | 23 (9-35) | 24 (17-52) | 0,246 |
| Swollen Joint Counts, n | 0 (0-2) | 0 (0-2) | 1 (0-2) | 0,480 |
| Tender Joint Counts, n | 2 (0-4) | 1 (0-4) | 2 (1-5) | 0,143 |
| CRP, mg/dl | 4 (3-13,65) | 3,14 (3-13) | 5 (3-19) | 0,107 |
| HAQ | 0,63 (0,25-1) | 0,63 (0,25-1) | 0,75 (0,63-0,94) | 0,097 |
| VAS-Physicians | 20 (12-31,5) | 20 (12-26) | 30 (15-50) | 0,074 |
| VAS-Patient Global | 50 (27-70) | 50 (20-70) | 61,5 (46,5-70) | 0,342 |
| VAS-Patient Pain | 53 (28-75) | 50 (20-75) | 69 (49,5-75) | 0,122 |
| DAS-28-CRP | 3,35 (2,2-3,9) | 2,85 (2-3,8) | 3,6 (2,9-4,4) | 0,086 |
| BASFI | 15 (8-27) | 14 (5-26) | 23,5 (12-30,5) | 0,133 |
| BASDAI | 28 (16-40) | 27,5 (14-36) | 39 (22,5-41,5) | 0,060 |
| ASDAS | 2,7 (1,9-3,2) | 2,6 (1,9-3,1) | 3 (2,35-3,4) | 0,122 |
| DAPSA-28 | 15,35 (7,2-21,9) | 14,9 (6,1-21,17) | 18,9 (14,15-29,3) | 0,108 |
Disclosure of Interests: None declared.