

Background: Pegloticase is indicated for treating uncontrolled gout, but some patients develop anti-drug antibodies that have been associated with a loss of efficacy and infusion reactions (IRs). 1 Per the pegloticase prescribing information, pre-infusion medications, including glucocorticoids (GCs), should be given to minimize IR risk. 2 Given that GCs have a significant side effect profile and patients with uncontrolled gout generally have multiple comorbidities, 3,4 minimizing steroid exposure is desirable.
Objectives: The current case series examines a community rheumatology practices’ experience with pre-infusion GC elimination in uncontrolled gout patients treated with pegloticase.
Methods: This retrospective chart review was conducted at a rheumatology practice that discontinues pre-infusion GC use in patients treated with pegloticase. Patients treated from 2016-2020 who had pre-infusion GCs discontinued during therapy were included. Pre-infusion prophylaxis at this practice includes methylprednisolone (125 mg IV), diphenhydramine (50 mg oral + 12.5 mg IVP), famotidine (40 mg oral), and acetaminophen (1000 mg) administered immediately before infusions. All other IR prophylaxis continued when GCs were stopped without tapering. Demographics, number of pegloticase infusions, and safety (adverse events [AEs], clinical lab values) were examined. Patients self-managed gout flares with oral methylprednisolone (24 mg then 5-day taper).
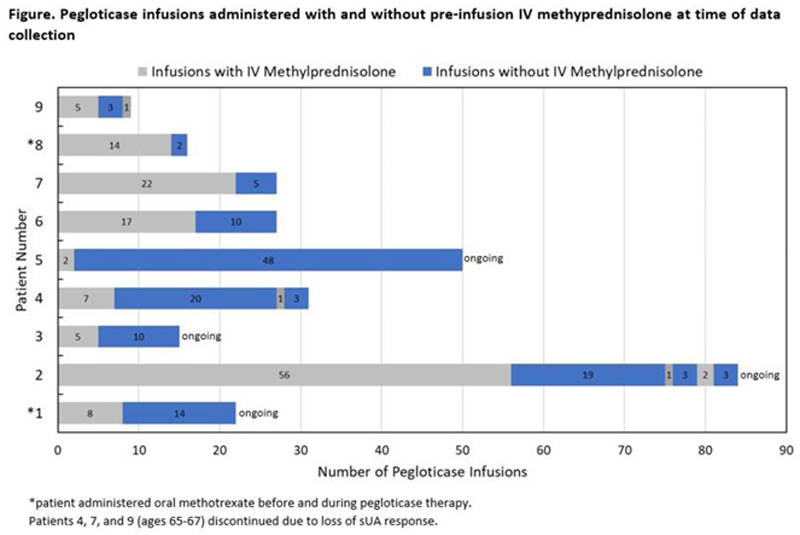
Results: Nine patients (8 male) with tophaceous gout met inclusion criteria. Mean age was 72.3 ± 6.9 years (65-84 years), mean BMI was 28.7 ± 5.6 kg/m 2 , and mean pre-therapy serum uric acid (sUA) was 7.3 ± 2.7 mg/dL (3.9-12.7 mg/dL). Two patients were co-treated with oral methotrexate (15 and 20 mg/week) and one patient was on hemodialysis. Most common comorbidities included osteoarthritis (9 patients), hypertension (8 patients), chronic kidney disease (5 patients), kidney stones (5 patients), and diabetes (5 patients). A median of 27 pegloticase infusions (range: 9-84) were administered, with 8 of 9 patients receiving ≥12 infusions. Median number of infusions before and after initial GC discontinuation was 7 (range: 2-56) and 10 (range: 2-48), respectively. Pre-infusion steroids were re-started in 3 patients after a rise in sUA, with 1 regaining sUA response (patient 2) and 2 discontinuing therapy (patients 4 and 9). An additional patient had a loss of sUA response (patient 7) and discontinued, 1 patient discontinued due to hospitalization (patient 6), and 1 patient chose to discontinue therapy (patient 8). At the time of data collection, 4 patients remained on therapy (patients 1-3, 5). Eight patients had at least one AE (all deemed unrelated to treatment), including influenza, hypertensive crisis, and toe amputation. No IRs occurred.
Conclusion: In this case series, 8 of 9 patients received 6 months or more (≥12 infusion) of pegloticase. These cases suggest that pre-infusion GC discontinuation may be possible in some patients treated with pegloticase (particularly those over 70 years). Further investigation exploring this concept is warranted, including evaluating the optimal time and conditions to discontinue pre-infusion GCs with pegloticase.
REFERENCES:
[1]Sundy JS, et al. JAMA. 2011;306(7):711-720.
[2]KRYSTEXXA (pegloticase) [prescribing information] Horizon.
[3]Saag KG. Bull NYU Hosp Jt Dis 2012;70(Suppl 1):S21-S25.
[4]Pillinger MH, et al. Bull NYU Hosp Jt Dis 2010;68:199-203.
Disclosure of Interests: Veronica Newsome Speakers bureau: Abbvie, Anthony Amatucci Shareholder of: Horizon Therapeutics plc, Employee of: Horizon Therapeutics plc, Tim Stainbrook: None declared., Brian LaMoreaux Shareholder of: Horizon Therapeutics plc, Employee of: Horizon Therapeutics plc.