

Background: Excessive and inappropriate production of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6 or IL-18, is a pathogenic cornerstone in adult and childhood onset Still’s disease. Beyond therapies targeting IL-1 or IL-6, Janus kinases (JAK) inhibitors have been proposed for adult-onset Still’s disease (AOSD) patients refractory to or intolerant of treatment with biologicals. Recently, it has been suggested that JAK inhibitors might be efficient in refractory AOSD patients 1 .
Objectives: To assess the efficacy and safety of JAK inhibitors in the treatment of refractory systemic juvenile idiopathic arthritis (sJIA) or AOSD.
Methods: This retrospective study was based on a national survey of the departments of rheumatology, paediatric rheumatology and internal medicine in all French hospitals from an online call of the “Club Rhumatismes et Inflammation” (
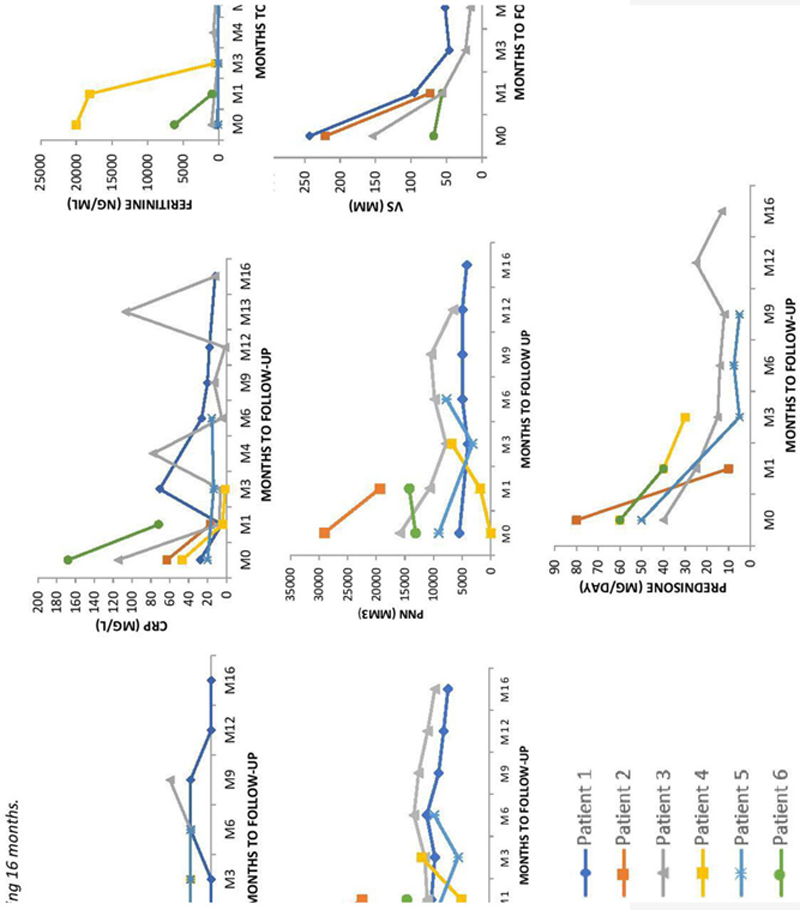
Results: 6 patients (5 adults and 1 child) were recruited (
Conclusion: JAK inhibitors therapy may be helpful for some patients with refractory Still’s disease. However, no complete response was observed in this short series of cases. There might be a difference of response between the molecules, although the number of patients is too low to draw conclusions. Additional information is thus needed to evaluate more precisely the risk-benefit ratio of this treatment, and a possible difference in efficacy among the different groups of JAK inhibitors.
REFERENCES:
[1]Hu Q, Wang M, Jia J, et al. Ann Rheum Dis 2020;0:1–3. doi:10.1136/annrheumdis-2019-216
Characteristics of the AOSD patient
| No. | Sex | Age (year ) | Main symptoms | Treatments before JAKi onset | JAK inhibitors | Steroids at onset (mg/day ) | Concomitant treatment | Response at last F-U | Steroids at the end of F-U (mg/day ) | F-U (months ) |
| 1 | F | 6 | Fever, polyarthritis, rash | AINS, ANAKI, TOCI, CANAKI, ADA, THALI, INFLIX | RUXOLITINIB 5mgx2/day | 3 | 0 | P | 1 | 23 |
| 2 | M | 28 | Fever, polyarthritis, rash | ANAKI | BARICITINIB 4mg/day | 80 | 0 | N | 10 | 1 |
| 3 | M | 32 | Fever, polyarthritis, rash | TOCI+MTX, ANAKI+MTX, CANAKI+MTX, ADA, CICLO, IgIV | BARICITINIB 4mg/day | 16 | MTX 20 mg/week ANAKINRA 100mg/day | P | 12 | 19 |
| 4 | F | 40 | Fever, polyarthritis, rash | MTX, IMUREL, CICLO, ETANERCEPT, ANAKI+MTX, TOCI+MTX, IgIV | RUXOLITINIB 15mgx2/day | 60 | ANAKI 200mg/day | P | 30 | 4 |
| 5 | F | 48 | Fever, polyarthritis, rash | TOCI, ANAKI, CICLO, CANAKI, IMUREL | TOFACITINIB 5mgx2/day | 50 | 0 | P | 7.5 | 9 |
| 6 | F | 50 | Fever, polyarthralgia, rash | ANAKI | BARICITINIB 4mg/day | 60 | 0 | N | 40 | 1 |
F-U : Follow-up
N : No response
P : Partial response
Acknowledgements: I thank all the coauthors, particularly Stéphane Mitrovic and Bruno Fautrel. Also, a special thank to the CRI.
Disclosure of Interests: None declared