

Background: Granulocyte/macrophage-colony stimulating factor (GM-CSF) is a cytokine both vital to lung homeostasis and important in regulating inflammation and autoimmunity 1,2,3 that has been implicated in the pathogenesis of respiratory failure and death in patients with severe COVID-19 pneumonia and systemic hyperinflammation. 4-6 Mavrilimumab is a human anti GM-CSF receptor α monoclonal antibody capable of blocking GM-CSF signaling and downregulating the inflammatory process.
Objectives: To evaluate the effect of mavrilimumab on clinical outcomes in patients hospitalized with severe COVID-19 pneumonia and systemic hyperinflammation.
Methods: This on-going, global, randomized, double-blind, placebo-controlled seamless transition Phase 2/3 trial was designed to evaluate the efficacy and safety of mavrilimumab in adults hospitalized with severe COVID-19 pneumonia and hyperinflammation. The Phase 2 portion comprised two groups: Cohort 1 patients requiring supplemental oxygen therapy without mechanical ventilation (to maintain SpO 2 ≥92%) and Cohort 2 patients requiring mechanical ventilation, initiated ≤48 hours before randomization. Here, we report results for Phase 2, Cohort 1: 116 patients with severe COVID- 19 pneumonia and hyperinflammation from USA, Brazil, Chile, Peru, and South Africa; randomized 1:1:1 to receive a single intravenous administration of mavrilimumab (10 or 6 mg/kg) or placebo. The primary efficacy endpoint was proportion of patients alive and free of mechanical ventilation at Day 29. Secondary endpoints included [1] time to 2-point clinical improvement (National Institute of Allergy and Infectious Diseases COVID-19 ordinal scale), [2] time to return to room air, and [3] mortality, all measured through Day 29. The prespecified evidentiary standard was a 2-sided α of 0.2 (not adjusted for multiplicity).
Results: Baseline demographics were balanced among the intervention groups; patients were racially diverse (43% non-white), had a mean age of 57 years, and 49% were obese (BMI ≥ 30). All patients received the local standard of care: 96% received corticosteroids (including dexamethasone) and 29% received remdesivir. No differences in outcomes were observed between the 10 mg/kg and 6 mg/kg mavrilimumab arms. Results for these groups are presented together. Mavrilimumab recipients had a reduced requirement for mechanical ventilation and improved survival: at day 29, the proportion of patients alive and free of mechanical ventilation was 12.3 percentage points higher with mavrilimumab (86.7% of patients) than placebo (74.4% of patients) (Primary endpoint; p=0.1224). Mavrilimumab recipients experienced a 65% reduction in the risk of mechanical ventilation or death through Day 29 (Hazard Ratio (HR) = 0.35; p=0.0175). Day 29 mortality was 12.5 percentage points lower in mavrilimumab recipients (8%) compared to placebo (20.5%) (p=0.0718). Mavrilimumab recipients had a 61% reduction in the risk of death through Day 29 (HR= 0.39; p=0.0726). Adverse events occurred less frequently in mavrilimumab recipients compared to placebo, including secondary infections and thrombotic events (known complications of COVID-19). Thrombotic events occurred only in the placebo arm (5/40 [12.5%]).
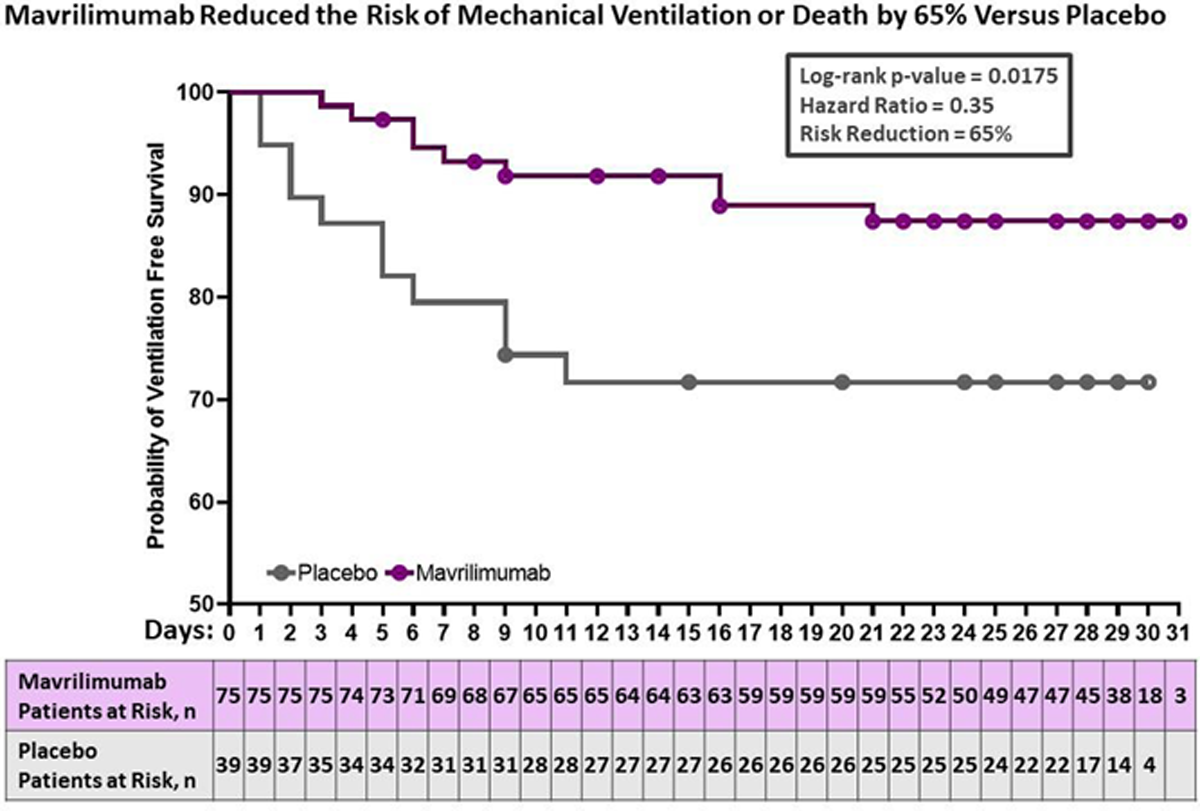
Conclusion: In a global, diverse population of patients with severe COVID-19 pneumonia and hyperinflammation receiving supplemental oxygen therapy, corticosteroids, and remdesivir, a single infusion of mavrilimumab reduced progression to mechanical ventilation and improved survival. Results indicate mavrilimumab, a potent inhibitor of GM-CSF signaling, may have added clinical benefit on top of the current standard therapy for COVID-19. Of potential importance is that this treatment strategy is mechanistically independent of the specific virus or viral variant.
REFERENCES:
[1]Trapnell, Nat Rev Dis Pri, 2019
[2]Wicks, Nat Rev Immunology, 2015
[3]Hamilton, Exp Rev Clin Immunol, 2015
[4]De Luca, Lancet Rheumatol, 2020
[5]Cremer, Lancet Rheumatol, 2021
[6]Zhou, Nature, 2020
Disclosure of Interests: Lara Pupim Employee of: Kiniksa, Shareholder of: Kiniksa, Tisha S. Wang Consultant of: Partner Therapeutics; steering committee for Kinevant BREATHE clinical trial, Kristin Hudock: None declared, Joshua Denson: None declared, Nyda Fourie: None declared, Luis Hercilla Vasquez: None declared, Kleber Luz: None declared, Mohammad Madjid Grant/research support from: Kiniksa, Kirsten McHarry: None declared, José Francisco Saraiva: None declared, Eduardo Tobar: None declared, Teresa Zhou Employee of: Kiniksa, Shareholder of: Kiniksa, Manoj Samant Employee of: Kiniksa, Shareholder of: Kiniksa, Joseph Pirrello Employee of: Kiniksa, Shareholder of: Kiniksa, Fang Fang Employee of: Kiniksa, Shareholder of: Kiniksa, John F. Paolini Employee of: Kiniksa, Shareholder of: Kiniksa, Arian Pano Employee of: Kiniksa, Shareholder of: Kiniksa, Bruce C. Trapnell: None declared