

Background: Dermatomyositis (DM) is a rare chronic systemic autoimmune disease with characteristic skin rash and progressive proximal muscle weakness. Current therapies encompass corticosteroids and other immunosuppressants and intravenous immunoglobulins (IVIg), however, none of these therapies are proven by randomized controlled phase 3 studies. There have been no large randomized clinical trials supporting the efficacy and safety of IVIg in DM.
Objectives: The ProDERM study aimed to evaluate the efficacy and safety/tolerability of IVIg in DM patients in a double-blind, randomized, placebo-controlled, international multi-center, phase III clinical trial.
Methods: The trial consisted of a double-blind, placebo-controlled First Period (16 weeks), in which adult patients with definite or probable DM (according to Bohan and Peter criteria) were randomized 1:1 to either high dose IVIg (2g/kg every 4 weeks) or placebo. Patients on placebo and patients without clinical worsening while on IVIg treatment entered the open label Extension Period (24 weeks) and received 2g/kg IVIg infusions every 4 weeks. To be included, subjects must have active disease with a manual muscle testing-8 (MMT-8) score < 142/150. Patients who showed clinical worsening (defined according to Oddis et al, 2013 - with slight adaptation) at 2 consecutive visits between week 8 and week 16 were switched to the alternate treatment arm.
Primary endpoint was the proportion of responders in the IVIg vs. placebo arm at week 16, where response was defined per 2016 ACR/EULAR Myositis response criteria of at least minimal improvement [Total Improvement Score (TIS) ≥ 20 points)] and without clinical worsening at 2 consecutive visits up to week 16.
Results: A total of 95 adult DM patients (mean age: 53 years; 75% females; 92% Caucasian) were enrolled, with 47 and 48 randomized to IVIg and placebo, respectively. Baseline clinical characteristics (including medical history and prior DM medication) were balanced between the 2 arms.
The study met the primary endpoint at week 16, with the proportion of responders being significantly higher in the IVIg group (37/47; 78.7%) as compared to the placebo group (21/48; 43.8%; p-value 0.0008;
Total Improvement Score – Analysis of Proportion of Responders at Week 16 (Full Analysis Set, N=95)
| TIS Response |
octagam 10%
|
Placebo
| Difference octagam 10% – placebo |
| Number (%) of responders | 37 (78.72%) | 21 (43.75%) | |
| Difference in response rates | 34.97 | ||
| [95% CI] p-value a | [16.70, 53.24] 0.0008 |
a Cochran-Mantel-Haenszel TestCI=confidence interval; N=number of patients; TIS=total improvement score
In the analysis of responders per improvement category at Week 16, a 45.2% higher response rate for at least moderate improvement (TIS ≥n40 points; p < 0.0001) and a 23.6% higher response rate for at least major improvement (TIS ≥060 points; p < 0.0062) was observed in the IVIG group as compared to the placebo group.
The mean (SD) TIS was significantly higher in IVIg group [48.4 (24.4)] than in placebo arm [21.6 (20.2)] at week 16 (
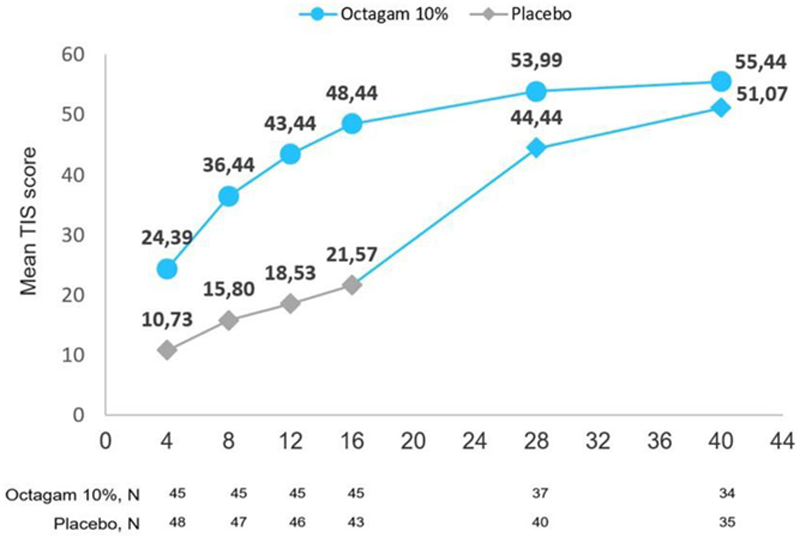
After switching to IVIG in the Extension Period the placebo group attained a similar response rate at Week 40 as did the IVIg treated patients at Week 16, i.e approx. 70% for minimal improvement.
In line with the overall primary endpoint, secondary end points including all of the sub-components of TIS except muscle enzyme (MMT-8, MD global, Extramuscular global, patient global, HAQ,) as well as CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index), also showed statistically significant improvement under IVIg treatment compared to placebo treatment.
The safety and tolerability profile of IVIg was consistent with previously reported safety outcomes for IVIg administration.
Conclusion: This is the first large international phase III randomized, placebo-controlled trial demonstrating the efficacy and safety of IVIg as a treatment for patients with DM.
REFERENCES:
[1]Oddis, C. V. et al. Arthritis Rheum (2013), 65, 314–324
Acknowledgements: Acknowledgments to all participating investigators, centers and patients and their families
Disclosure of Interests: Rohit Aggarwal Consultant of: Q32, Alexion, Argenx, AstraZeneca, BMS, Boehringer Ingelheim, Corbus, Csl Behring, EMD Serono, Janssen, Kezar, Mallinckrodt, Kyverna, Octapharma, Orphazyme, Pfizer., Grant/research support from: BMS, Mallinckrodt, Pfizer, EMD Serono, Christina Charles-Schoeman Consultant of: Pfizer, Abbvie, Octapharma, Gilead, Regeneron-Sanofi, Grant/research support from: Bristol Myers Squibb, Pfizer, Abbvie, Octapharma, Joachim Schessl Speakers bureau: Octapharma, Grifols, CSL Behring, Consultant of: Octapharma, Zsuzsanna Bata-Csorgo Speakers bureau: Novartis, Sanofi-Genzyme, Ewopharma, Consultant of: Sanofi-Genzyme, Novartis, Ewopharma, Mazen Dimachkie Consultant of: ArgenX, Catalyst, Cello, CSL-Behring, EcoR1, Kezar, Momenta, NuFactor, Octapharma, RaPharma/UCB, RMS Medical, Sanofi Genzyme, Shire Takeda, Spark Therapeutics and UCB Biopharma., Grant/research support from: Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol-Myers Squibb, Catalyst, Corbus, CSL-Behring, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, Novartis, Octapharma, Orphazyme, Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, UCB Biopharma, Viromed/Healixmith., Zoltán Griger Speakers bureau: Abbvie, CSL-Behring, Eli-Lilly, Roche, Boehringer Ingelheim, Consultant of: Octapharma, Sergey Moiseev: None declared, Chester V Oddis Consultant of: EMD Serono; Alexion Pharmaceuticals, Inc, Grant/research support from: Genentech (Clinical trial support); Bristol Myers Squibb (Clinical trial support), Elena Schiopu Consultant of: Octapharma, Grant/research support from: Octapharma, Janssen (Johnson & Johnson), BMS, Pfizer, Abbvie, Jirˇí Vencovský Speakers bureau: Abbvie, Biogen, MSD, Pfizer, Roche, Sanofi, UCB, Consultant of: Abbvie, Boehringer, Eli Lilly, Octapharma, Gilead, Irene Beckmann Employee of: Octapharma, Todd Levine Shareholder of: Corinthian Reference Labs, CND Life Sciences, Consultant of: Grifols, Octapharma, Alexion, Elisabeth Clodi Employee of: Octapharma PPG, Vienna Austria, and the ProDERM Investigators: None declared