

Background: BAFF and APRIL are TNF superfamily members that form homo- and heteromultimers that bind TACI and BCMA on B cells; BAFF also binds BAFF-R. BAFF and APRIL support B cell development, differentiation, and survival, particularly for plasmablasts and plasma cells, and play critical roles in the pathogenesis of B cell-related autoimmune diseases. In nonclinical models, inhibition of either BAFF or APRIL alone mediates relatively modest effects, whereas their co-neutralization dramatically reduces B cell function, including antibody production. Fc fusions of wild-type (WT) TACI (e.g. atacicept and telitacicept) target both BAFF and APRIL and have demonstrated promising clinical potential in e.g. systemic lupus erythematosus (SLE) and IgA nephropathy but have not yet clearly exhibited long-term and/or complete disease remissions.
Objectives: To generate a dual BAFF/APRIL antagonist with inhibitory activity superior to WT TACI and BCMA and with the potential to improve clinical outcomes in B cell-mediated diseases.
Methods: Our directed evolution platform was used to identify a potent variant TNFR domain (vTD) of TACI that exhibits significantly enhanced affinity for BAFF and APRIL as compared to WT TACI; this TACI vTD domain was fused to a human IgG Fc to generate the therapeutic candidate ALPN-303. ALPN-303 was evaluated for functional activity in: 1) human lymphocyte assays, 2) the NOD. Aec1Aec2 spontaneous model of Sjogren’s syndrome (SjS), 3) the bm12-induced mouse model of lupus, 4) the (NZB/NZW)F 1 spontaneous model of lupus, and 5) preclinical rodent and cynomolgus monkey pharmacokinetic/pharmacodynamic studies.
Results: ALPN-303 inhibited BAFF- and APRIL-mediated signaling
in vitro
in human lymphocyte assays, with significantly lower IC
50
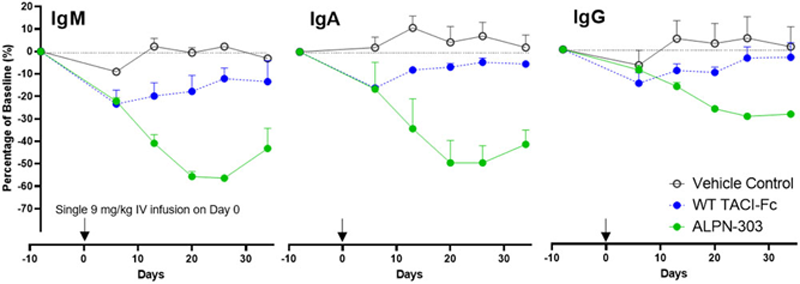
values than WT TACI-Fc and belimumab comparators. In all mouse models evaluated, administration of ALPN-303 rapidly and significantly reduced key lymphocyte subsets including plasma cells, germinal center B cells, and follicular T helper cells. ALPN-303 significantly reduced autoantibodies and sialadenitis in the spontaneous SjS model, inhibited glomerular IgG deposition in the bm12-induced model of lupus, and potently suppressed anti-dsDNA autoAbs, blood urea nitrogen levels, proteinuria, sialadenitis, kidney lesions, and renal immune complex deposition in the NZB/W lupus model. As compared to WT TACI-Fc, ALPN-303 exhibited higher serum exposure and significantly and persistently decreased titers of serum IgM, IgG, and IgA antibodies in mice and cynomolgus monkeys (
ALPN-303 induces more potent suppression, as compared to WT TACI-Fc, of serum immunoglobulins following a single 9 mg/kg IV infusion (on Day 0; arrows) in female cynomolgus monkeys.
Conclusion: ALPN-303 is a potent BAFF/APRIL antagonist derived from our directed evolution platform that consistently demonstrates encouraging immunomodulatory activity and efficacy in vitro and in vivo , superior in preclinical studies to anti-BAFF antibody and WT TACI-Fc. This novel Fc fusion molecule demonstrates favorable preliminary developability characteristics, including higher serum exposures and more potent immunosuppressive activities, which may enable lower clinical doses and/or longer dosing intervals than WT TACI-Fc therapeutics. ALPN-303 may thus be an attractive development candidate for the treatment of multiple autoimmune and inflammatory diseases, particularly B cell-related diseases such as SLE, SjS, and other connective tissue diseases. Preclinical development is underway to enable the initiation of clinical trials later this year.
Disclosure of Interests: Stacey R. Dillon Shareholder of: Alpine Immune Sciences, Bristol Myers Squibb, Employee of: Alpine Immune Sciences, Bristol Myers Squibb, Lawrence S. Evans Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Katherine E. Lewis Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Jing Yang Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Mark W. Rixon Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Joe Kuijper Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Dan Demonte Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Janhavi Bhandari Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Steve Levin Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Kayla Kleist Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Sherri Mudri Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Susan Bort Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Daniel Ardourel Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Michelle A. Seaberg Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Rachel Wang Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Chelsea Gudgeon Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Russell Sanderson Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Martin F. Wolfson Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Jan Hillson Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences, Stanford L. Peng Shareholder of: Alpine Immune Sciences, Employee of: Alpine Immune Sciences