

Background: Ocular involvement is a relatively frequent and potentially severe complication of sarcoidosis. Oral corticosteroids (OCS) are the first-line treatment. Conventional immunosuppressive agents (cIS) and biological therapy (BT) can be used in refractory cases (1-5).
Objectives: To evaluate the treatment and visual outcomes of a cohort of patients diagnosed with ocular sarcoidosis.
Methods: Study of a large cohort (n=384) of all consecutive patients diagnosed with sarcoidosis from January 1, 1999 to December 31, 2019 at a single University Hospital. Finally, 344 patients were included according the ATS/ERS/WASOG criteria (Eur Respir J. 1999; 14:735-7). Different ocular manifestations and the following systemic treatments were assessed: a ) OCS, b ) cIS), c ) monoclonal TNF inhibitors, d ) Etanercept (ETN), e ) Tocilizumab (TCZ). Best Corrected Visual Acuity (BCVA) according to different systemic treatments was compared at diagnosis and after one year of follow-up (Kruskall Wallis test).
Results: 344 patients were reviewed. From these, 65 (18.9%) presented ocular manifestations as uveitis (83.1%), orbital lesions (7.7%), retinal vasculitis (6.2%), dry eye (6.2%) and scleritis (1.5%). All of them received systemic treatment. BT was particularly used in patients with retinal vasculitis (100%), panuveitis (75%) and orbital lesions (40%). Systemic treatment and BCVA outcome according to ocular manifestations are shown in table. Median BCVA at onset and after one year was 0.6 [interquartile range (IQR) 0.3-0.8] and 0.9 [0.6-1], respectively. No statistically significant differences were observed between systemic treatments in BCVA of patients with uveitis after 1 year of follow-up (
Median [25,75 IQR] BVCA after one year follow up according to type of systemic treatment in sarcoid uveitis.
Median BCVA after one year Systemic treatment
Abbreviations: BCVA: Best Corrected Visual Acuity; OCS: Oral Corticosteroids; Conventional IS: Conventional immunosupressants; Monoclonal TNFi: monoclonal tumour necrosis factor inhibitors; ETN: Etanercept; TCZ: Tocilizumab.
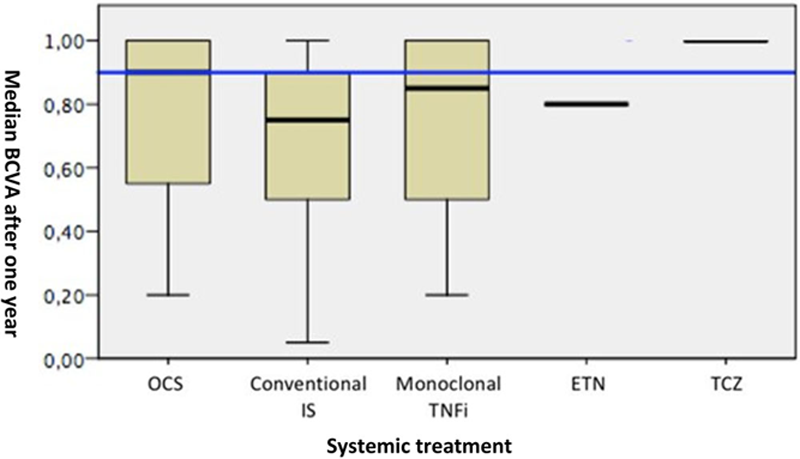
Conclusion: Panuveitis, intermediate uveitis and orbital lesions, require a more aggressive treatment than other manifestations of ocular sarcoidosis. In uveitis, an important improvement in BCVA after 1 year of follow-up was observed regardless of the type of treatment used.
REFERENCES:
[1]Riancho-Zarrabeitia L, et al. Semin Arthritis Rheum 2015;45(3):361-8. PMID: 26092330
[2]Riancho-Zarrabeitia L et al. Clin Exp Rheumatol 2014;32(2):275-84. PMID: 24321604
[3]Vegas-Revenga N, et al. Am J Ophthalmol 2019; 200:85-94. PMID: 30660771
[4]Cordero-Coma et al. Mediators Inflamm 2014; 2014:717598. PMID: 24976689
[5]Calvo-Río V, et al. Clin Exp Rheumatol 2014; 32 (4 Suppl 84): S54-7. PMID: 25005576
Median BCVA at onset and after one year according to ocular manifestations and type of systemic therapy.
| Type of ocular affectation | n (% ) | Median BCVA at onset [IQR] | Median BCVA after 1 year [IQR] |
OCS
|
cIS
|
monoclonal TNFi
|
ETN
|
TCZ
|
| Uveitis | 54 (83.1) | 0.6 [0.3-0.8] | 0.9 [0.6-1] | 44 (81.5) | 29 (53.7) | 16 (29.6) | 3 (5.5) | 3 (5.5) |
| - Anterior | 31 (47.7) | 0.7 [0.3-0.8] | 0.8 [0.5-1] | 22 (70.9) | 12 (38.7) | 2 (6.5) | 2 (6.5) | 0 |
| - Intermediate | 2 (3.1) | 0.5 | 0.7 | 2 (100) | 1 (50) | 1 (50) | 1 (50) | 1 (50) |
| - Posterior | 5 (5.2) | 0.5 [0.1-0.9] | 0.9 [0.9-1] | 4 (80) | 4 (80) | 3 (60) | 0 | 0 |
| - Panuveitis | 16 (24.6) | 0.4 [0.2-0.7] | 0.9 [0.5-1] | 16 (100) | 12 (75) | 10 (62.5) | 0 | 2 (12.5) |
| Orbital lesions | 5 (7.7) | 0.5 [0.1-0.6] | 1 [0.1-1] | 4 (80) | 2 (40) | 2 (40) | 0 | 1 (20) |
| Retinal vasculitis | 4 (6.2) | 0.6 [0.5-0.8] | 1 [0.6-1] | 4 (100) | 4 (100) | 1 (25) | 0 | 1 (25) |
| Dry eye | 4 (6.2) | 1 | 0.9 | 2 (50) | 1 (25) | 0 | 0 | 0 |
| Scleritis | 1 (1.5) | 1 | 1 | 1 (100) | 0 | 0 | 0 | 0 |
Abbreviations: BCVA: Best Corrected Visual Acuity; IQR: Interquartile Range; OCS: Oral Corticosteroid; cIS: Conventional Immunosuppressants; Monoclonal TNFi: monoclonal tumour necrosis factor inhibitors; ETN: Etanercept; TCZ: Tocilizumab.
Disclosure of Interests: Carmen Álvarez-Reguera: None declared, Jorge Javier Gaitán-Valdizán: None declared, Raúl Fernández-Ramón: None declared, Rosalía Demetrio-Pablo: None declared, José Luis Martín-Varillas: None declared, Lara Sanchez-Bilbao: None declared, David Martínez-López: None declared, Iñigo González-Mazón: None declared, Miguel Á. González-Gay Speakers bureau: Abbvie, Pfizer, Roche, Sanofi and MSD., Grant/research support from: Abbvie, MSD, Janssen and Roche., Ricardo Blanco Speakers bureau: Abbvie, Pfizer, Roche, Bristol-Myers, Janssen, Lilly and MSD., Grant/research support from: Abbvie, MSD and Roche.