Background: In clinical trials as well as in real-life, the IL-1ß inhibitor canakinumab leads to rapid remission of symptoms in the treatment of CAPS, a monogenic autoinflammatory disease with severe systemic and organ inflammation.
Objectives: The RELIANCE registry is designed to explore long-term safety and effectiveness of canakinumab under routine clinical practice conditions in pediatric (≥2 years) and adult patients with CAPS, including Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndrome (FCAS), and neonatal onset multisystem inflammatory disease (NOMID)/chronic infantile neurological cutaneous and articular syndrome (CINCA).
Methods: This prospective, non-interventional, observational study with a 3-year follow-up enrolls patients with clinically confirmed diagnoses of CAPS routinely receiving canakinumab. In 6-monthly visits, clinical data, physician assessments and patient-reported outcomes are evaluated starting at baseline with last update at 30 months of follow-up in the total cohort including the cohort with severe CAPS subtypes (NOMID/CINCA).
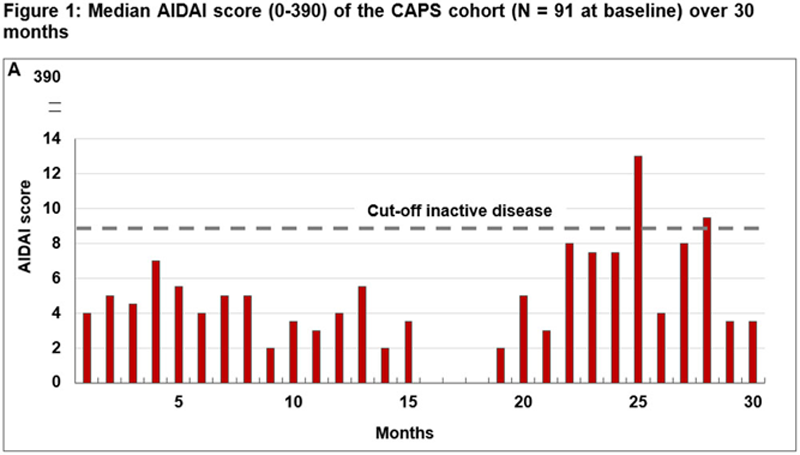
Results: 91 CAPS patients (50% female; 14 [15%] NOMID/CINCA subtypes) were enrolled by December 2020 (
Patient and physician assessment of clinical CAPS disease activity and laboratory markers over time.
| Baseline | 12 months | 30 months | ||||
| Total cohort | NOMID/ CINCA | Total cohort | NOMID/ CINCA | Total cohort | NOMID/ CINCA | |
| Number of patients, N | 91 | 14 | 67 | 8 | 28 | 4 |
| Number (%) of patients with days absent from work/school during last 6 months | 30 (34) | 4 (29) | 28 (42) | 2 (25) | 17 (61) | 4 (100) |
| Number (%) of patients in disease remission (physician assessment) | 61 (68.5) | 11 (78.6) | 42 (66.7) | 4 (66.7) | 19 (67.9) | 4 (100.0) |
| Physician Global Assessment, percentage of absent/mild-moderate/severe rating, % | 40 / 53 / 2 | 57 / 36 / 0 | 33 / 60 / 2 | 33 / 50 / 0 | 61 / 39 / 0 | 75 / 25 / 0 |
| Patient assessment of current disease activity; 0–10, median (min; max) | 2.0 (0; 7) | 1.0 (0; 6) | 1.0 (0; 7) | 1.0 (0; 5) | 0.0 (0; 7) | 0.0 (0; 4) |
| Patient assessment of current fatigue; 0–10, median (min; max) | 3.0 (0; 9) | 2.0 (0; 6) | 3.0 (0; 8) | 2.0 (0; 8) | 1.0 (0; 8) | 4.0 (0; 5) |
| Number (%) of patients without impairment of social life by the disease | 32 (52.5) | 4 (50.0) | 31 (62.0) | 3 (42.9) | 11 (47.8) | 1 (33.3) |
| CRP, median (mg/dl) | 0.1 | 0.2 | 0.1 | 0.5 | 0.0 | 0.2 |
| SAA, median (mg/dl) | 0.3 | 0.4 | 0.5 | 0.9 | 0.3 | 0.1 |
| ESR, median (mm/h) | 5.0 | 6.0 | 5.0 | 3.5 | 5.0 | 5.5 |
| SAE | Number of events | Incidence rate per 100 patient years | ||||
| Total cohort | NOMID/CINCA | Total cohort | NOMID/CINCA | |||
| All types of SAE | 39 | 3 | 14.72 | 11.72 | ||
| SADR | 20 # | 0 | 10.69 | 0.00 | ||
# Alport’s syndrome, appendicitis, blister, cardiovascular disorder, chest pain, circulatory collapse, erythema, febrile convulsion, glomerulonephritis, haemophilus test positive, pneumonia, premature delivery, skin discolouration, tonsillectomy, tonsillitis bacterial, tonsillitis streptococcal (all N=1 event), pyrexia (N=3 events), not yet coded (inpatient admission, N=1 event) CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; n. a., not annotated; SAA, serum amyloid A; SADR, serious adverse drug reaction; SAE, serious adverse event
Conclusion: The 30-month interim analysis of the RELIANCE study demonstrates that long-term canakinumab treatment is safe and effective in patients with any subtype of CAPS. However, impairment of social life and days off school/work still exists.
Disclosure of Interests: J. B. Kuemmerle-Deschner Consultant of: Novartis, AbbVie, Sobi, Grant/research support from: Novartis, AbbVie, Sobi, Birgit Kortus-Goetze Consultant of: Novartis, Prasad Oommen Grant/research support from: Novartis, Ales Janda: None declared, Jürgen Rech Speakers bureau: bbvie, Biogen, BMS, Chugai, GSK, Janssen, Lilly, MSD; Mylan, Novartis, Roche, Sanofi, Sobi, UCB, Consultant of: Abbvie, Biogen, BMS, Chugai, GSK, Janssen, Lilly, MSD, Mylan, Novartis, Roche, Sanofi, Sobi, UCB, Grant/research support from: Novartis, Sobi, Tilmann Kallinich Consultant of: Sobi, Novartis, Roche, Grant/research support from: Novartis, Frank Weller-Heinemann: None declared, Gerd Horneff Speakers bureau: AbbVie, Bayer, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Grant/research support from: AbbVie, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Ivan Foeldvari Consultant of: Novartis, Catharina Schuetz: None declared, Michael Borte Grant/research support from: Pfizer, Shire, Axel Braner Consultant of: Novartis and SOBI, Julia Weber-Arden Employee of: Novartis, Norbert Blank Consultant of: Novartis, Sobi, Lilly, Pfizer, Abbvie, BMS, MSD, Actelion, UCB, Boehringer-Ingelheim, Roche, Grant/research support from: Novartis, Sobi