

Background: Various hypotheses exist for the explanation of PLC response rates in RA clinical trials. Here we hypothesized that PLC-treated patients who continue to take methotrexate (cMTX) when entering the trial compared to those who have no cMTX would have higher response rates, because of increased adherence to MTX in the trial environment.
Objectives: To compare differences in response rates in PLC treated RA patients receiving MTX background therapy to patients receiving PLC without background conventional synthetic (cs)DMARDs in two RCTs.
Methods: To investigate the hypothesis we conducted a post-hoc analysis of two RCTs that allowed inclusion of patients with and without cMTX - the GO-AFTER and the SIRROUND-T trials, investigating golimumab and sirukumab, respectively, compared with PLC in patients who had an insufficient response to biological DMARDs.(1,2)
All PLC randomized patients of both trials were pooled and included in the analyses; we did not analyse the active treatment groups. Subsequently we stratified the pooled PLC group into patients receiving PLC on top of cMTX and patients receiving PLC without any csDMARD as background therapy. We compared American College of Rheumatology (ACR) 20/50/70% response rates and Clinical Disease Activity Index (CDAI) low disease activity (LDA, i.e. CDAI≤10) responses between the two groups using Fisher’s exact test.
Similar to the primary analyses of the individual studies, non-responder imputation (NRI) for state outcomes (ACR responses, CDAI LDA) was applied in patients who initiated any csDMARD after randomization, had MTX or glucocorticoid doses increased during the study or discontinued the study. NRI was also applied for early escape patients receiving active therapy after failing to achieve a ≥20% decrease in joint counts at week (wk) 16 in GO-AFTER and at wk 18 in SIRROUND-T.
Results: Of the 398 pooled PLC patients, 285 continued their MTX and 113 had no MTX (or other background csDMARDs). Baseline characteristics were similar (
Baseline characteristics
| Placebo without background csDMARD | Placebo + continued MTX | |
| n | 113 | 285 |
| Age (years) * | 56 (48-64) | 56 (45-64) |
| Female (% ) | 95 (84.1%) | 236 (82.8%) |
| Disease duration (years ) | 8.4±5.2 | 8.8±7.5 |
| ACPA positive (% ) | 88 (77.9%) | 214 (75.1%) |
| RF positive (% ) | 90 (79.6%) | 211 (74%) |
| SJC 66 (0-66 ) | 15.9±9.9 | 16.2±10.8 |
| TJC 68 (0-68 ) | 29.1±16.8 | 26.7±15.7 |
| PGA (VAS 0-10 ) | 6.8±2.2 | 6.5±2.1 |
| EGA (VAS 0-10 ) | 6.4±1.8 | 6.1±1.9 |
| Pain (VAS 0-10 ) | 7.0±1.9 | 6.6±2.1 |
| HAQ-DI (0-3 ) | 1.6±0.7 | 1.6±0.6 |
| CRP (mg/dL) * | 1.2 (0.5-3.5) | 1.0 (0.4-2.6) |
| CDAI | 39.4±13.5 | 38.5±12.8 |
| Concomitant GC intake (% ) | 67 (59.3%) | 174 (61.1%) |
| Concomitant MTX (% ) | 0 (0%) | 285 (100%) |
| MTX dosage ≥12.5mg (% ) | 0 (0%) | 222 (77.9%) |
| MTX dosage <12.5mg (% ) | 0 (0%) | 63 (22.1%) |
Data is shown as mean (± standard deviation) or n (%) unless stated otherwise
* median (IQR)
At wk 16, an ACR20 response was achieved by 72/285 (25.3%) of PLC+cMTX and 14/113 (12.4%) receiving PLC only patients (p=0.005); for ACR50 these numbers were 25/285 (8.4%) vs. 1/113 (0.9%; p=0.003); and for ACR70 they were 8/285 (2.8%) vs. 0/113 (0%; p=0.112). Also, significantly more PLC+cMTX patients achieved a CDAI LDA at wk 16 (25/285, 8.8%) compared to PLC only treated patients (2/113; 1.8%; p=0.013). Results between the two arms were numerically or statistically different already from week 4 (for ACR 20) or week 8 (ACR 50, CDAI LDA) onwards (
ACR 20/50/70 and CDAI LDA responses of PLC patients with continued MTX vs. PLC patients without csDMARD background therapy.
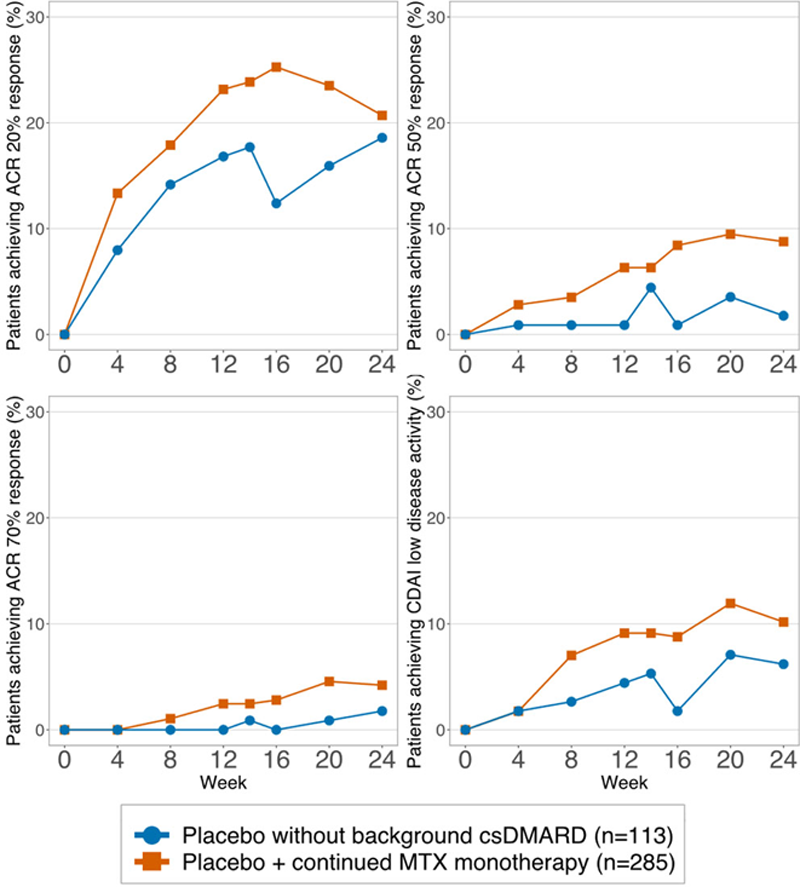
Conclusion: In patients randomized to placebo therapy, continued MTX background therapy increases clinical responses and achievement of good clinical states. These findings imply that pre-existing and putatively insufficient background therapy should be effectively optimized before enrollment into a clinical trial protocol.
REFERENCES:
[1]Smolen JS et al. Lancet 2009; 374 : 210–221.
[2] Aletaha D et al. Lancet 2017; 389 : 1206–1217.
Acknowledgements: This study, carried out under YODA Project # 2018-3704, used data obtained from the Yale University Open Data Access Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C.
Disclosure of Interests: Andreas Kerschbaumer Speakers bureau: AbbVie, Bristol-Myers Squibb, Celgene, Eli-Lilly, Gilead, Merck Sharp and Dohme, Novartis and Pfizer, Paid instructor for: Celgene, Zaida Iasha Rivai: None declared, Josef S. Smolen Speakers bureau: AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO Pharma, Janssen, Lilly, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi, and UCB, Consultant of: AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Gilead, ILTOO Pharma, Janssen, Lilly, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi, and UCB, Grant/research support from: Abbvie, AstraZeneca, Janssen, Lilly, Merck Sharpe & Dohme, Pfizer, and Roche, Daniel Aletaha Speakers bureau: Abbvie, Amgen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, Consultant of: Abbvie, Amgen, Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, Grant/research support from: Abbvie, Amgen, Lilly, Novartis, Roche, SoBi, Sanofi