

Background: The presence of sacroiliitis in patients (pts) with ankylosing spondylitis (AS) on imaging can be established both by sacroiliac joint (SIJ) X-ray or MRI. Active sacroiliitis on MRI as defined by ASAS is predictor of good treatment response to biological disease modifying anti-rheumatic drugs [1, 2]. Netakimab (NTK) is a humanized anti-interleukin-17A antibody approved for the treatment of AS, psoriatic arthritis, moderate-to-severe plaque psoriasis in Russia and Belarus. The difference in treatment response to NTK in AS pts with and without active sacroiliitis on MRI (MRI+/MRI−) is unclear.
Objectives: To report the changes in AS activity in pts with and without sacroiliitis on MRI at week 16 of NTK treatment.
Methods: ASTERA (NCT03447704) is an ongoing phase 3 placebo (PBO)-controlled clinical study, aimed at evaluating NTK efficacy in AS. All pts fulfilled modified New York criteria. Evaluation of acute inflammation on SIJ MRI was performed at the baseline but was not an inclusion criterion. This analysis includes pts received subcutaneous NTK 120 mg every 2 wks with available baseline SIJ MRI. The presence of sacroiliitis on MRI was defined as SPARCC>2. Efficacy endpoints included ASAS20/40, ASAS partial remission (PR), changes from baseline in BASDAI (Bath Ankylosing Spondylitis Disease Activity Index), ASDAS-CRP (Ankylosing Spondylitis Disease Activity Score).
Results: 67 MRI+ and 46 MRI− pts were included into analysis. Baseline characteristics were balanced across both arms. 42.4% of MRI+ pts and 38.3% of MRI− pts achieved ASAS40 at week 16 (p≥0.05), ASAS20 was observed in 65.2%/55.3% pts in the same arms respectively (p≥0.05). ASAS PR was reported for 15.2% MRI+ and 17.0% MRI− pts (p≥0.05). Improvements in BASDAI and ASDAS-CRP were similar across both arms. At wk 16, mean change from baseline in BASDAI was −2.7 vs −3.0 for MRI+ and MRI− pts respectively, mean change in ASDAS-CRP was −1.7 vs −1.4 in the same arms (p≥0.05 for all), (
Clinical improvements in AS disease activity. Mean change from baseline is shown for (A) ASDAS-CRP, and (B) BASDAI
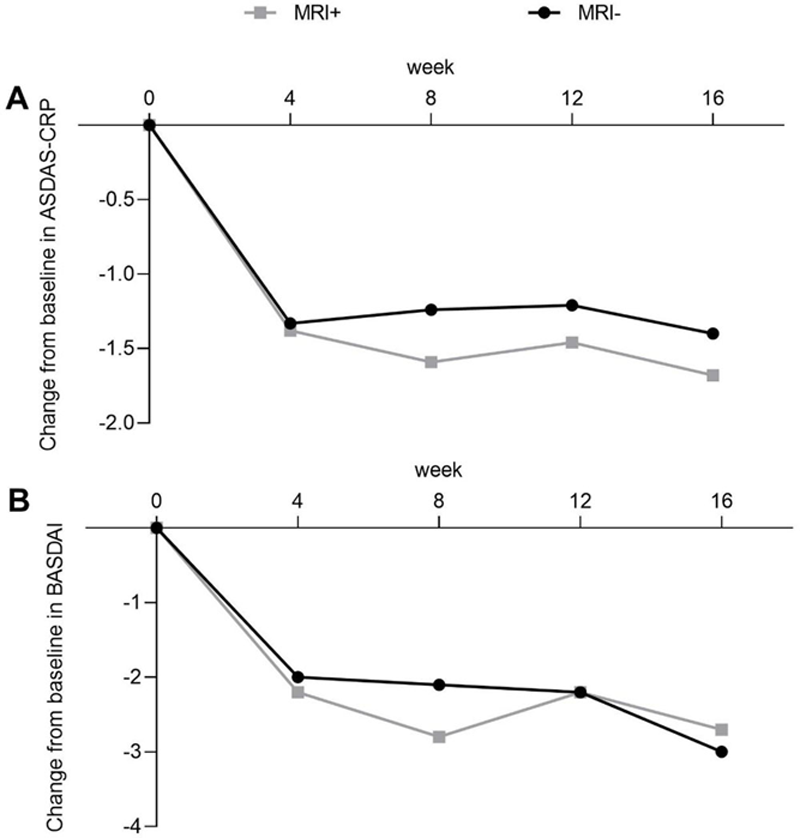
Conclusion: NTK leads to decline of disease activity in AS pts irrespectively of sacroiliitis on MRI.
REFERENCES:
[1]Rudwaleit M, et al. MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis. 2008;67(9):1276-81.
[2]Sieper J, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015;67(10):2702-12.
Acknowledgements: This study was sponsored by JSC BIOCAD.
Disclosure of Interests: Inna Gaydukova Speakers bureau: Abbvie, Biocad, Eli Lilly, MSD, Novartis, Pfizer, Sandoz, V Mazurov: None declared, Shandor Erdes: None declared, Tatiana Dubinina: None declared, Alena Kundzer: None declared, Nikolaj Soroka: None declared, Anna Eremeeva Employee of: Biocad