Background: Enthesitis is a key clinical domain and imaging hallmark of psoriatic arthritis (PsA). Ultrasound (US) is a highly sensitive tool for detecting synovitis and enthesitis in PsA. The Outcome Measures in Rheumatology Initiative (OMERACT) has developed an US definition and scoring system of enthesitis for clinical studies. 1 The ULTIMATE study (NCT02662985) is the first large double-blind (DB), placebo-controlled phase IIIb study designed to demonstrate a rapid and significant benefit of subcutaneous secukinumab vs. placebo on US detected synovitis in patients with PsA. 2
Objectives: To report the enthesitis response to secukinumab over 24 weeks using two novel US composite enthesitis scores.
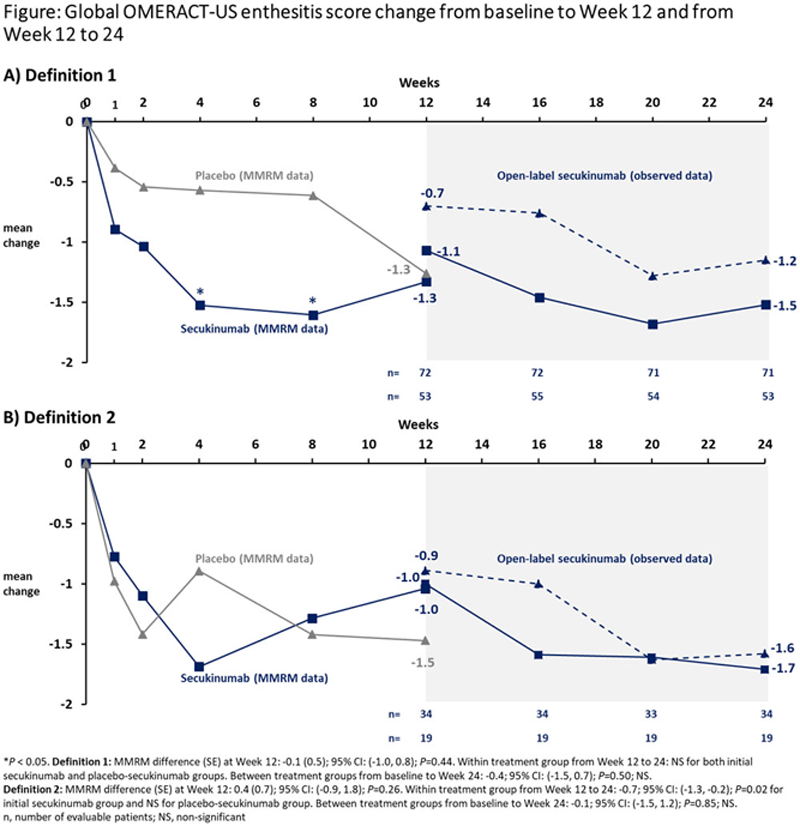
Methods: This was a 52-week study consisting of a 12-week DB, a 12-week open-label (OL) and a 6-month extension period. 2 Inclusion criteria required ≥1 clinical enthesitis as per SPARCC enthesitis index, but not US-assessed enthesitis. 2 Patients were randomised (1:1) to either weekly secukinumab (300 or 150 mg according to severity of skin psoriasis) or placebo followed by 4-weekly dosing thereafter. All placebo patients switched to OL secukinumab (placebo-secukinumab) at Week 12. Throughout the study, enthesitis was assessed with SPARCC and US. Six anatomical sites were assessed bilaterally with US: insertions of lateral epicondyle tendons, quadriceps, patellar ligaments (distal and proximal insertions), Achilles tendons and plantar fascia. Two exploratory global OMERACT-US enthesitis scores were tested: Definition 1 combining power Doppler (PD; 0–3) and Grey Scale (0–1) inflammation and Definition 2 rating PD only (0–3) across the six anatomical sites. Data were analysed with mixed-effect model repeated measures (MMRM) up to Week 12 and as observed from Week 12 to 24. The comparison of OMERACT-US enthesitis score within treatment groups was tested with paired and between treatment groups with unpaired t-tests.
Results: Of 166 patients enrolled, 93% completed 24 weeks of treatment (secukinumab, 95%; placebo-secukinumab, 92%). The average clinical enthesitis count at baseline was 4. Since the presence of PD was not a mandatory inclusion criterion, a higher proportion of patients met Global OMERACT-US enthesitis score with Definition 1 vs. Definition 2 (81% vs. 33%) at baseline (
Distribution of US detected enthesitis at baseline according to OMERACT enthesitis score Definition 1 and 2
| Secukinumab | Placebo | |||
| Def 1 >0 | Def 2 >0 | Def 1 >0 | Def 2 >0 | |
| N= | 73 | 34 | 61 | 20 |
| Anatomical sites, % | ||||
| Achilles tendon | 49 | 12 | 45 | 2 |
| Lateral epicondyle | 49 | 21 | 46 | 21 |
| Patellar ligament distal insertion | 34 | 8 | 29 | 4 |
| Patellar ligament proximal insertion | 34 | 10 | 18 | 4 |
| Plantar fascia | 36 | 0 | 28 | 0 |
| Quadriceps insertion | 55 | 12 | 40 | 2 |
Proportion of patients is irrespective of the enthesitis site left or right side. N, total number of patients
Conclusion: A consistent clinical and US response on enthesitis was shown through 24 weeks across initial secukinumab and placebo switcher groups. While ULTIMATE has demonstrated the responsiveness of these global OMERACT-US enthesitis scores, further work is required to test these scores in PsA cohorts with inclusion criteria for both clinical and US enthesitis.
REFERENCES:
[1]Balint PV, et al. Ann Rheum Dis . 2018;77:1730-5.
[2]D’Agostino MA, et al. Arthritis Rheumatol . 2020;72(suppl 10).
Disclosure of Interests: Maria-Antonietta D’Agostino Speakers bureau: Sanofi, Novartis, BMS, Janssen, Celgene, AbbVie, UCB pharma and Eli Lilly, Consultant of: Sanofi, Novartis, BMS, Janssen, Celgene, AbbVie, UCB pharma and Eli Lilly, Philip G Conaghan Speakers bureau: AbbVie, AstraZeneca, BMS, Eli Lilly, Galapagos, Gilead, Novartis and Pfizer, Consultant of: AbbVie, AstraZeneca, BMS, Eli Lilly, Galapagos, Gilead, Novartis and Pfizer, Corine Gaillez Shareholder of: Novartis and BMS, Employee of: Novartis, Maarten Boers Consultant of: BMS, Novartis, Pfizer, and GSK, Esperanza Naredo Speakers bureau: AbbVie, Roche, BMS, Pfizer, UCB, Eli Lilly, Novartis, Janssen and Celgene, Consultant of: AbbVie, Novartis and BMS, Grant/research support from: Eli Lilly, Philippe Carron Speakers bureau: Pfizer, MSD, Novartis, BMS, AbbVie, UCB, Eli Lilly, Gilead and Celgene, Consultant of: Pfizer, MSD, Novartis, BMS, AbbVie, UCB, Eli Lilly, Gilead and Celgene, Grant/research support from: UCB, MSD and Pfizer, Petra Hanova: None declared, Tomás Cazenave: None declared, Catherine Bakewell Speakers bureau: AbbVie, Novartis, Sanofi Genzyme, and consulting honoraria from Pfizer, UCB, and Janssen, Consultant of: AbbVie, Novartis, Sanofi Genzyme, and consulting honoraria from Pfizer, UCB, and Janssen, Anne-Marie Duggan Employee of: Novartis, Punit Goyanka Employee of: Novartis, Georg Schett Speakers bureau: AbbVie, BMS, Celgene, Gilead, Janssen, Eli Lilly, Novartis, Roche and UCB pharma