Background: Guselkumab (GUS), a selective monoclonal antibody targeting the interleukin-23p19 subunit, has demonstrated efficacy in 2 pivotal Ph3 psoriatic arthritis (PsA) studies (DISCOVER-1, 1 DISCOVER-2 2 ).
Objectives: Evaluate GUS efficacy and safety in PsA patients (pts) with inadequate response (IR) to tumor-necrosis-factor inhibition (TNFi) through Week24 (W24) of the Ph3b COSMOS study.
Methods: In this randomized, double-blind, placebo (PBO)-controlled trial, 285 pts with active PsA (≥3 swollen & ≥3 tender joints) who demonstrated lack of benefit or intolerance to 1-2 TNFi were randomized 2:1 to subcutaneous GUS 100mg (n=189) or PBO (n=96) at W0, W4, then every 8 weeks (Q8W) through W44 (with PBO crossover to GUS at W24). At W16, pts who met early escape (EE) criteria (<5% improvement in both tender & swollen joint counts) also could switch from PBO to GUS. The primary efficacy endpoint was ACR20 response at W24 among randomized, treated pts. Pts missing ACR20 data at W24 or who met treatment failure criteria (including meeting EE criteria at W16) were considered nonresponders (NRs). Subgroup analyses were performed to assess consistency of primary treatment effect based on demographics, disease characteristics, and medication use at baseline. Prespecified sensitivity analyses included ‘Per-Protocol’ (PP) (excluded pts with major protocol deviations) and ‘EE-Correction’ (included pts incorrectly routed to EE) analyses. Adverse events (AEs) were summarized by treatment received.
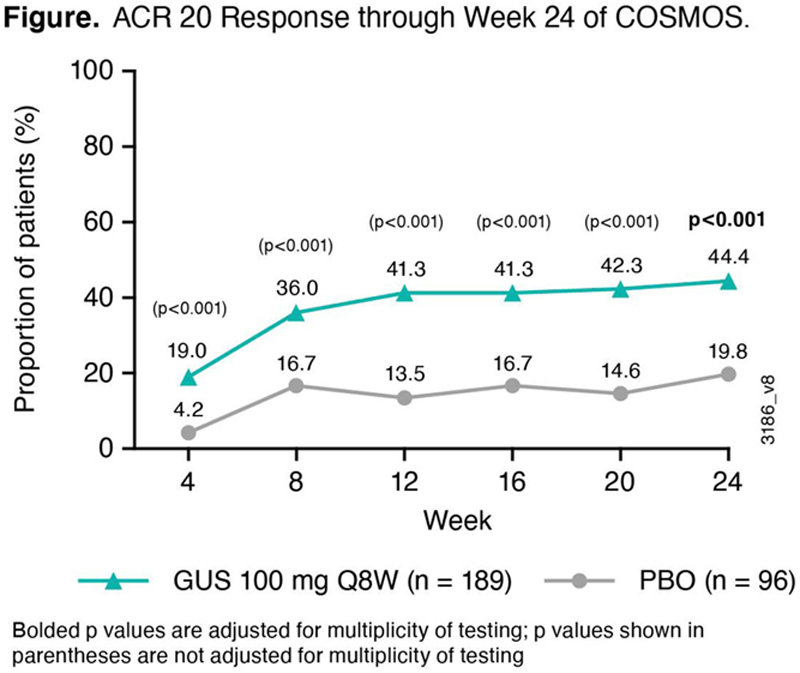
Results: Baseline characteristics were similar across GUS and PBO pts, though a higher proportion of females and more severe joint symptoms were seen in the GUS group. At W24, 44.4% of GUS vs 19.8% of PBO pts achieved ACR20 (p<0.001) (
Baseline characteristics of, and adverse events reported by, randomized and treated COSMOS pts
| GUS 100 mg Q8W (N=189 ) | PBO (N=96 ) | |
| Age, y | 49 | 49 |
| Sex, Female | 54% | 46% |
| Duration of PsA, y | 8.3 | 8.7 |
| Body mass index, kg/m 2 | 29 | 31 a |
| Swollen (0-66) / tender (0-68) joint count | 10 / 21 | 9 / 18 |
| Pt pain / Pt global arthritis / Physician global disease, 0-10 cm VAS | 6.5 / 6.5 / 6.9 | 6.0 / 6.2 / 6.4 |
| Health Assessment Questionnaire-Disability Index, 0-3 | 1.3 b | 1.2 |
| C-reactive protein, mg/dL | 1.2 b | 1.2 |
| Methotrexate use at baseline | 56% | 53% |
| Psoriatic body surface area, % | 17.9 | 13.4 |
| Number of prior TNFi: 1 / 2 | 88% / 12% | 89% / 11% |
| Reason for prior TNFi discontinuation: Efficacy / Safety | 84% / 16%* | 81% / 19%* |
| Pts with ≥1 AE / SAE | 37% / 3% | 48% / 3% |
| Pts with ≥1 infection / serious infection | 18% / 0% | 20% / 0% |
| Pts with ≥1 AE leading to study agent discontinuation | 2% | 2% |
| Pts with ≥1 malignancy | 0.4% | 0 |
| Pts with ≥1 injection-site reaction | 2% | 1% |
Data shown are mean or %. a N=95; b N=188. *Missing for 1 pt. SAE – serious adverse events ; VAS – visual analog scale
Conclusion: In this Ph3b, placebo-controlled study of PsA pts with IR to 1-2 TNFi, GUS 100 mg Q8W elicited a significantly higher ACR20 response rate vs. PBO at W24; results of prespecified sensitivity and subgroup analyses were consistent. GUS safety in TNF-IR PsA pts through W24 is consistent with the favorable GUS safety profile in psoriasis and biologic-naïve PsA pts. 3
REFERENCES:
[1]Deodhar A. Lancet 2018;391: 2213–24.
[2]Mease PJ. Lancet 2020;395: 1126–36.
[3]Guselkumab Prescribing Information. Janssen Biotech, Inc.
Disclosure of Interests: Laura C Coates Consultant of: AbbVie, Amgen, Biogen, BMS, Boehringer Ingelehim, Celgene, Domain, Eli Lilly, Gilead, Janssen, Medac, Novartis, Pfizer and UCB, Grant/research support from: AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Novartis, Pfizer, Laure Gossec Consultant of: AbbVie, Amgen, BMS, Biogen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis, UCB, Grant/research support from: Amgen, Eli Lilly, Galapagos, Janssen, Pfizer, Sandoz, Sanofi, Elke Theander Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, Paul Bergmans Shareholder of: Johnson & Johnson, Employee of: Janssen, Marlies Neuhold Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, Chetan Karyekar Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, Wim Noel Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, Georg Schett: None declared, Iain McInnes Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, and UCB