

Background: The presence of myositis specific anti-melanoma differentiation associated protein 5 (MDA5) autoantibodies is associated with mucocutaneous ulcerations, rapidly progressing interstitial lung disease (RPILD), arthritis and mild muscle involvement in patients. RPILD is the major cause of mortality. At present it is unknown which domain of the MDA5 protein is the main elicitor of an immunogenic response.
Objectives: The aim of this study is to delineate the domains in the MDA5 protein that are the target of autoantibodies.
Methods: Anti-MDA5 IgG were isolated from MDA5(+) patient plasma (7 UPMC, 1 KI and 1 KULeuven) by affinity chromatography using an in-house affinity column as described earlier in
Ossipova et al, 2014
(1)
. 8 constructs covering different regions of the MDA5 protein were recombinantly produced in
E.coli
(Uniprot ID Q9BYX4,
Graphical presentation of the constructs representing different (combinations of) domains of the MDA5 protein.
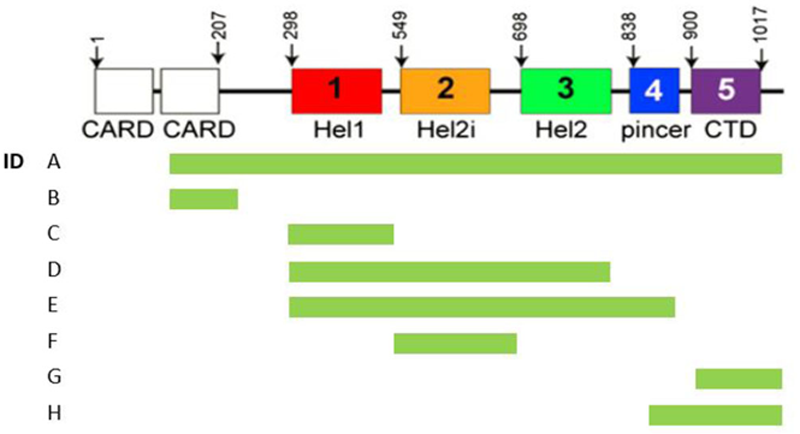
Results: The preliminary data suggest the main reactivity of the plasma samples and the corresponding purified autoantibodies is directed towards the helicase domains and that there is variability between the patients in the reactivity towards domains located at the end of the protein.
Conclusion: The study aims to resolve the main immunogenic domain of the MDA5 protein, which will lead to more insight in the disease mechanisms. The preliminary results suggest this domain is in the center of the MDA5 protein, but further experiments are necessary. We will use this set up to study differences in reactivity between patients (from different cohorts) and assess if differences in antibody reactivity could be linked to clinical features such as RPILD. Such correlations might be beneficial to predict the disease progression and to apply personal treatment approaches.
REFERENCES:
[1]Ossipova E, Cerqueira CF, Reed E, Kharlamova N, Israelsson L, et al. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis Res Ther. 2014;16(4):R167.
[2]Fernandes-Cerqueira C, Renard N, Notarnicola A, Wigren E, Gräslund S, et al. Patients with anti-Jo1 antibodies display a characteristic IgG Fc-glycan profile which is further enhanced in anti-Jo1 autoantibodies. Scientific reports. 2018;8(1):17958.
Disclosure of Interests: Eveline Van Gompel: None declared, Catia Cerqueira: None declared, Edvard Wigren: None declared, Susanne Gräslund: None declared, Karine Chemin: None declared, Begum Horuluoglu: None declared, Ellen De Langhe: None declared, Olivier Benveniste: None declared, Ingrid E. Lundberg Consultant of: Consulting fees from Corbus Pharmaceuticals, Inc, Grant/research support from: Research grants from Bristol Myers Squibb and AstraZeneca.