

Background: Psoriatic arthritis (PsA), a chronic inflammatory disease characterized by peripheral arthritis, axial inflammation, dactylitis, enthesitis & skin/nail psoriasis, causes impaired physical function, disability & loss of work productivity.
Objectives: Evaluate associations between PsA clinical characteristics & outcomes including fatigue & work productivity using Work Productivity & Activity Impairment Questionnaire: PsA (WPAI-PsA).
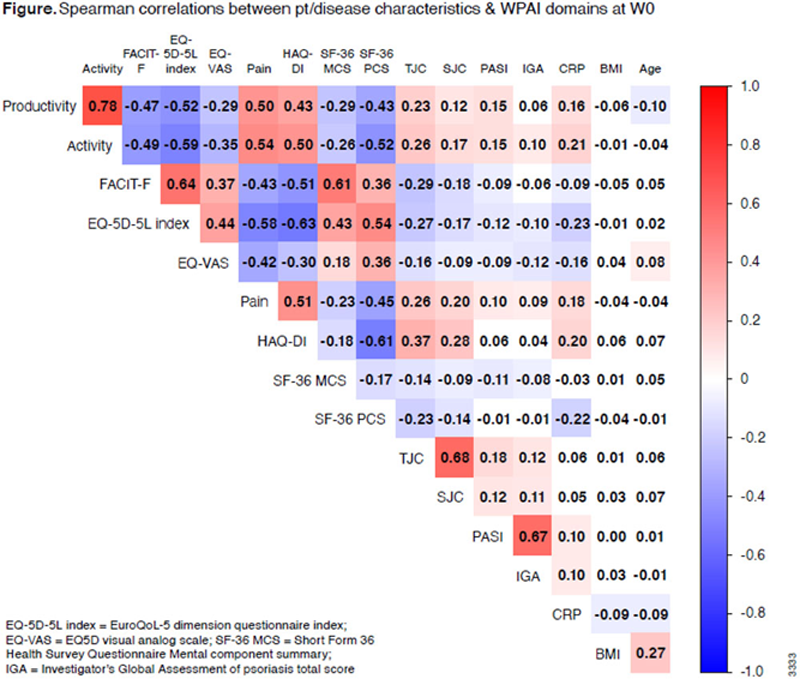
Methods: The Phase 3 DISCOVER-2 trial assessed guselkumab (GUS), an anti-IL-23p19 subunit monoclonal antibody, in bio-naïve adults with active PsA (swollen joint count [SJC] ≥5 & tender joint count [TJC] ≥5, C-reactive protein [CRP] ≥0.6 mg/dL) despite standard therapies. 1 Patients (Pts) were randomized 1:1:1 to GUS 100 mg Q4W; GUS 100 mg at W0, W4, then Q8W; or placebo (PBO). WPAI-PsA assesses PsA-related work time missed (absenteeism), impairment while working (presenteeism), productivity loss (absenteeism+presenteeism), & daily activity during the previous week. Spearman correlation testing evaluated relationships between pt demographics & disease characteristics of PsA & WPAI domain scores based on observed values at baseline. Univariate linear regression assessed associations between WPAI & these variables based on observed data at W0 & at W24. Variables with p<0.10 were included in a multivariate analysis employing a mixed-effects model for repeated measures, controlling for all other variables; resulting p-values <0.05 were considered statistically significant.
Results: As reported elsewhere,
2
least-squares mean % changes from baseline at W24 were -3.8/-19.5/-20.0/-20.5 for GUS Q4W, -3.1/-19.4/-19.7/-21.5 for GUS Q8W, & -3.5/-10.2/-10.9/-10.3 for PBO for absenteeism, presenteeism, absenteeism+presenteeism, & daily activity impairment, respectively. Among 738 pts, WPAI domain scores were moderately to strongly correlated (ie, ≥0.4) with pt-reported pain (0-10 visual analog scale), physical function (Health Assessment Questionnaire Disability Index [HAQ-DI]), fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F] scale) & 36-Item Short Form Health Survey (SF-36) Physical Component Summary (PCS) score, but weakly correlated with other variables (
Conclusion: In PsA pts, extra-articular symptoms, fatigue, pain & elevated CRP were significantly associated with WPAI-assessed work & activity impairment. Treating all major clinical manifestations of PsA is needed to help pts improve work & activity impairment. GUS effectively treats all major clinical manifestations 1 & improves work & activity impairment in PsA. 2
REFERENCES:
[1]Mease P. Lancet 2020;395:1126-36.
[2]Curtis J. ACR 2020; Poster 0332.
Multivariate analysis of clinical characteristics/outcomes & WPAI domains at W0 & W24
| Parameter | Absenteeism a | Presenteeism a | Productivity Loss a | Activity Impairment b | ||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Age | -0.05 | 0.42 | -0.27 | <0.001 | -0.28 | <0.001 | -0.06 | 0.17 |
| Female | 0.91 | 0.46 | -1.54 | 0.22 | -1.74 | 0.20 | 2.38 | 0.02 |
| CRP | 0.73 | 0.04 | 0.97 | 0.01 | 1.01 | 0.01 | 0.89 | <0.001 |
| FACIT-F | -0.31 | <0.001 | -0.67 | <0.001 | -0.73 | <0.001 | -0.75 | <0.001 |
| Pain | 1.03 | <0.001 | 4.15 | <0.001 | 4.25 | <0.001 | 4.02 | <0.001 |
| PASI | 0.06 | 0.36 | 0.16 | 0.02 | 0.14 | 0.05 | 0.15 | 0.003 |
| SJC | 0.08 | 0.48 | -0.05 | 0.61 | -0.05 | 0.66 | 0.03 | 0.75 |
| TJC | -0.10 | 0.13 | 0.11 | 0.09 | 0.09 | 0.19 | 0.10 | 0.04 |
| Dactylitis (Y/N) | -1.10 | 0.39 | 2.47 | 0.05 | 2.58 | 0.05 | 0.54 | 0.57 |
| Enthesitis (Y/N) | 1.52 | 0.20 | 2.38 | 0.04 | 2.99 | 0.01 | 2.40 | 0.01 |
a Pts working at baseline
b All pts in study
Disclosure of Interests: Jeffrey Curtis Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Eli Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Eli Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB, Iain McInnes Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and UCB, Dafna D Gladman Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB, Feifei Yang Shareholder of: Janssen, Employee of: Janssen, Steve Peterson Shareholder of: Janssen, Employee of: Janssen, Prasheen Agarwal Shareholder of: Janssen, Employee of: Janssen, Alexa Kollmeier Shareholder of: Janssen, Employee of: Janssen, Elizabeth C Hsia Shareholder of: Janssen, Employee of: Janssen, Chenglong Han Shareholder of: Janssen, Employee of: Janssen, May Shawi Shareholder of: Janssen, Employee of: Janssen, William Tillett Speakers bureau: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, MSD, Pfizer, and UCB, Grant/research support from: AbbVie, Celgene, Eli Lilly, Janssen, and Novartis, Philip J Mease Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, SUN, and UCB, Proton Rahman Speakers bureau: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB, Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB, Grant/research support from: Janssen and Novartis