

Background: Rheumatoid Arthritis (RA) is a systemic autoimmune disease with a prevalence of 0.5-1% worldwide 1 . Anti-cytokine antibodies, especially anti-Tumor Necrosis Factor (TNF) antibodies are considered the gold standard for RA therapy. However, there are some concerns regarding their lack of therapeutic efficacy in a significant proportion of patients 2 and their potential systemic implications such as the risk of serious infections 3 . Developing novel agents with synovial targeting specificity might help to increase the therapeutic index while reducing systemic side effects of RA therapeutics.
Objectives: Our work aims to develop a novel bispecific tandem single-chain variable fragment (scFv)-Fc fusion protein combining synovium specificity with anti-TNFα activity. The potential advantage of this construct is a reduced systemic TNF-binding activity and increase delivery and activation of the TNF-neutralising capacity at the inflamed joints.
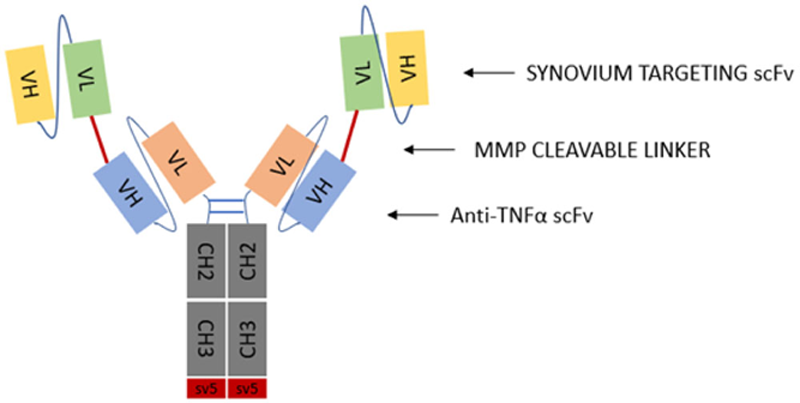
Methods: The therapeutic tandem scFv-Fc fusion protein comprises two external arms with synovium specific targeting ability linked through a metalloproteinase (MMP) cleavable linker to the anti-TNFα variable fragments of Adalimumab fused to the CH2 and CH3 domains of IgG1 (
Results: The fusion protein was tested by immunohistochemistry staining on RA synovium biopsies and an array of non-inflamed human tissues showing specific targeting of synovial microvasculature without no reactivity to the non-inflamed tissues. The TNFα binding and blocking capacity of the fusion protein was measured respectively by ELISA and cell assays measuring NF-κB activation, and it showed a two-fold decreased activity compared to the control antibody Adalimumab prior to detachment of the cleavable targeting fragment shielding the active anti-TNF fragment. Human synovial fluid and recombinant human MMP-1 efficiently cleaved the external arms of the antibody, releasing the anti-TNF scFv-Fc. The cleaved construct, detached from the synovium targeting arms, showed the same binding and anti-TNF inhibitory capacity/potency as Adalimumab.
Conclusion: The novel bispecific tandem scFv-Fc demonstrated specific synovium targeting ability and intended reduced anti-TNF activity in its intact form prior to reaching the joint. Following MMPs-induced cleavage present in RA synovial fluid the therapeutic activity was restored to the same level as Adalimumab. Overall, this construct has the potential of decreasing the anti-TNF off-site activity and consequently, reduce systemic toxicity while maintaining high on-site activity. Also, the presence of a synovium targeting domain has the advantage of increasing the delivery and retention within the inflamed synovium and possibly increase the therapeutic index of this anti-TNF therapeutics.
Schematic diagram of the bispecific tandem scFv-Fc fusion protein.
REFERENCES:
[1]Silman, A. J. & Pearson, J. E. Epidemiology and genetics of rheumatoid arthritis. 265–272 (2002).
[2]Balogh, E. et al. Comparison of remission criteria in a tumour necrosis factor inhibitor treated rheumatoid arthritis longitudinal cohort: patient global health is a confounder. Arthritis Res. Ther. 15 , R221 (2013).
[3]Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emp. Rheumatology (Oxford). 50 , 124–131 (2011).
[4]Kamperidis, P. et al. Development of a novel recombinant biotherapeutic with applications in targeted therapy of human arthritis. Arthritis Rheum. 63 , 3758–3767 (2011).
Disclosure of Interests: None declared