

Background: Biological disease modifying anti-rheumatic drugs (bDMARDs) and Janus Kinase inhibitors (JAKi) are both recommended post conventional synthetic disease modifying anti-rheumatic drug (csDMARD) therapy failure in active rheumatoid arthritis (RA), but the data on long-term durability are limited.
Objectives: The objective of this study is to analyze a database of patients at the Western University, Rheumatology Center who initiated a bDMARD or JAKi and compare the proportion and characteristics of patients associated with retention of a drug class.
Methods: This was a single-center study of 215 adult RA patients (82.76 % females, age 59.8 ± 12.0 years, disease duration 15.5 ± 10.0 years;
Results: In 215 patients, there were 320 treatment events (148 bDMARDs, 172 JAKi) and 142 discontinuations (53.5% bDMARDs, 46.5% JAKi).
Conclusion: EULAR guidelines have placed bDMARDs equal to JAKi as post csDMARD failure therapy in active RA. However, this study demonstrates that JAKi has a greater durability than biologics regardless of gender, age, disease duration, and line of therapy. Therefore, JAKi may be considered as a preferable method of treatment post csDMARD failure in active RA.
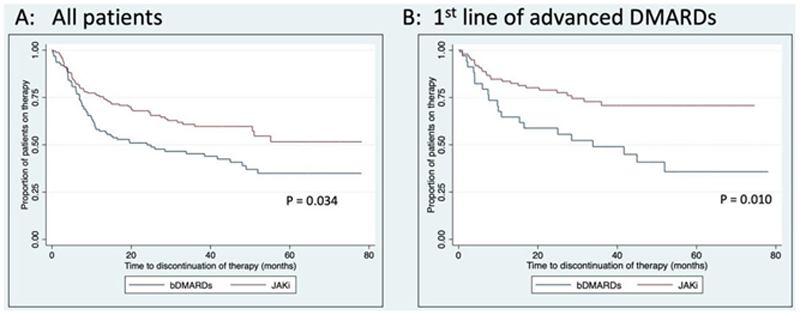
Kaplan-Meier survival curves for (A) time to discontinuation of therapy in all RA patients receiving bDMARDs versus JAKi; P-value represents Cox regression adjusted for gender, age, disease duration, and line of therapy (B) time to discontinuation of therapy in patients using bDMARDs/JAKi as first line of advanced therapy; P-value represents Cox regression adjusted for drug class, gender, age, and disease duration
Patient demographics and hazard ratios for discontinuation of bDMARDs versus JAKi by Cox regression model
| Characteristic | JAKi (N=172 ) | bDMARD (N=148 ) | Mean |
| Age (years) | 60.9 | 58.5 | 59.8 |
| Sex (% F) | 77.8 | 88.5 | 82.8 |
| Disease duration (years) | 15.3 | 15.8 | 15.5 |
| Line of advanced therapy (% first line) | 57.6 | 31.1 | 45.3 |
| Drug used (%) | Tofacitinib: 93.5 | Rituximab: 26.4 | |
| Etanercept: 19.6 | |||
| Adalimumab: 17.6 | |||
| Predictors of Drug Discontinuation | HR (95% CIs ) | P values | |
| Crude Model | JAKi vs bDMARDs | 0.60 (0.43, 0.84) | 0.003 |
| Adjusted model | JAKi vs bDMARDs | 0.68 (0.47, 0.97) | 0.034 |
| Male vs female | 0.77 (0.46, 1.31) | 0.342 | |
| Age | 1.01 (0.99, 1.03) | 0.123 | |
| RA duration | 0.99 (0.97, 1.01) | 0.500 | |
| Treatment line 1 vs >1 | 0.59 (0.40, 0.88) | 0.010 | |
Disclosure of Interests: Karla Machlab: None declared, Samir M. Iskandar: None declared, Tatiana Nevskaya: None declared, Louise Vanderhoek: None declared, Jillian Bylsma: None declared, Sara Hewitt: None declared, Janet Pope Speakers bureau: AbbVie, Amgen, BMS, BI, Gilead, Galapagos, Janssen, Lilly, Medexus, Merck, Novartis, Pfizer, Sanofi, Sandoz, Consultant of: AbbVie, Amgen, BMS, BI, Celltrion, Gilead, Galapagos, Janssen, Lilly, Medexus, Merck, Novartis, Pfizer, Roche, Samsung, Sanofi, Sandoz, Teva, UCB, Grant/research support from: AbbVie, BMS, Merck, Pfizer, Roche, Seattle Genetics