

Background: C-OPTIMISE was a phase 3b clinical trial investigating certolizumab pegol (CZP) maintenance dose continuation, reduction or withdrawal following achievement of sustained remission in patients with axial spondyloarthritis (axSpA). During the C-OPTIMISE maintenance period, the majority of patients randomised to CZP, either the full or reduced maintenance dose, did not experience disease flares. Conversely, in those who had CZP withdrawn, only a minority of patients remained flare-free. 1
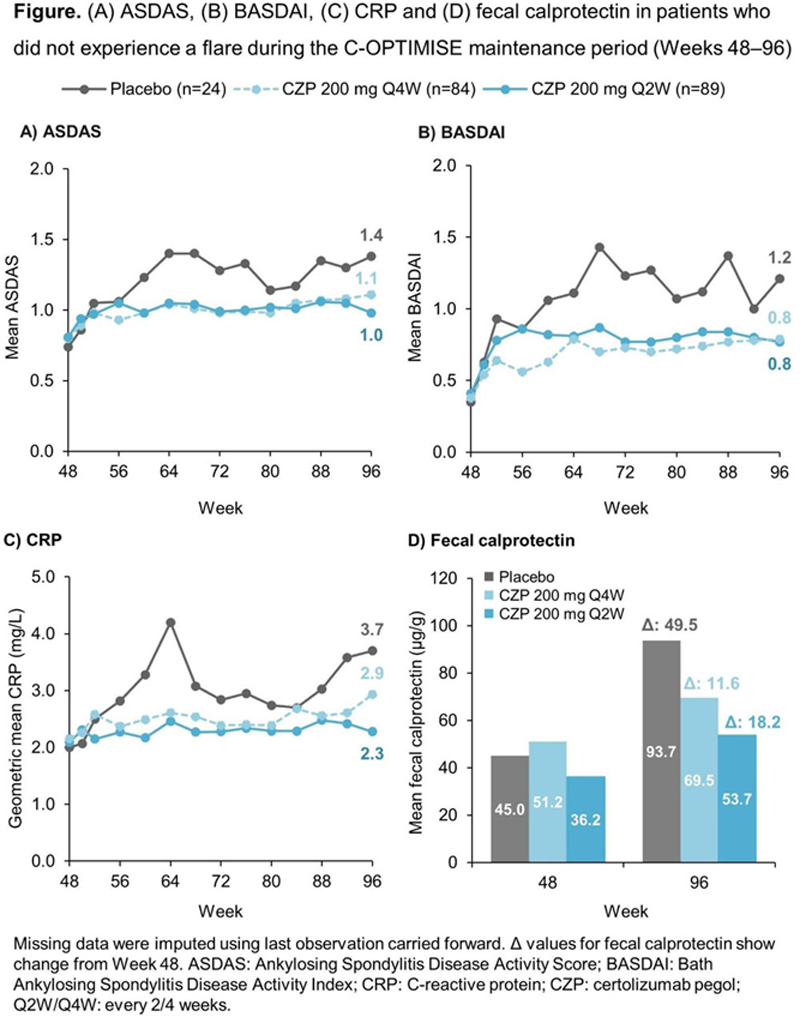
Objectives: This post-hoc analysis evaluates disease activity and clinical markers of inflammation in patients who did not experience a disease flare following randomisation to CZP full maintenance dose, CZP reduced maintenance dose or placebo (PBO) during the maintenance period (Weeks 48–96) of C-OPTIMISE.
Methods: C-OPTIMISE (NCT02505542) was a multicentre, double-blind, parallel-group, randomised phase 3b study with a 48-week open-label run-in period. 1 Adult patients with early (<5 years’ symptom duration) active axSpA received open-label CZP 200 mg every 2 weeks (Q2W) for the first 48 weeks; from Week 48, patients who achieved sustained remission (Ankylosing Spondylitis Disease Activity Score [ASDAS] <1.3 at Week 32 or 36 and Week 48) were randomised 1:1:1 to double-blind CZP 200 mg Q2W (full maintenance dose), CZP 200 mg Q4W (reduced maintenance dose) or PBO for a further 48 weeks (maintenance period). A flare was defined as ASDAS ≥2.1 at two consecutive visits or ASDAS >3.5 at any visit. We report ASDAS, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and C-reactive protein (CRP) and fecal calprotectin levels during Weeks 48–96 in CZP- and PBO-randomised patients who did not experience a flare (i.e. completed Week 96 on randomised treatment). Missing data were imputed using last observation carried forward.
Results: Of 313 patients entering the maintenance period at Week 48, 197 (62.9%) completed Week 96 on randomised treatment without experiencing a flare; of these, 89 (85.6%) and 84 (80.0%) patients were in the CZP 200 mg Q2W and CZP 200 mg Q4W arm, respectively, with only 24 (23.1%) patients randomised to PBO not experiencing a flare. Baseline characteristics of these patients are shown in the
Baseline (Week 0) characteristics of patients who did not experience flares during the C-OPTIMISE maintenance period
| Placebo
| CZP 200 mg Q4W (n=84) | CZP 200 mg Q2W (n=89) | |
| Age (years), mean (SD) | 29.8 (7.4) | 32.9 (6.7) | 32.4 (7.2) |
| Male, n (%) | 19 (79.2) | 69 (82.1) | 69 (77.5) |
| Time since diagnosis (years) | |||
| Mean (SD) | 2.0 (1.8) | 2.0 (1.7) | 2.5 (1.6) |
| Median | 1.2 | 1.2 | 2.7 |
| Symptom duration (years) | |||
| Mean (SD) | 2.7 (1.7) | 3.4 (1.9) | 3.9 (2.9) |
| Median | 2.8 | 3.5 | 3.9 |
| ASDAS, mean (SD) | 3.4 (0.8) | 3.7 (0.8) | 3.7 (0.7) |
| BASDAI, mean (SD) | 6.3 (1.1) | 6.6 (1.5) | 6.4 (1.4) |
| CRP (mg/L), geometric mean | 6.28 | 7.88 | 7.35 |
| Fecal calprotectin (µg/g), mean (SD) | 71.8 (111.4) | 87.1 (110.5) | 81.0 (120.0) |
SD: standard deviation.
Conclusion: Despite not meeting the threshold for a flare, consistently higher disease activity and increases in serologic and inflammatory biomarkers were observed in PBO-randomised patients who did not experience a flare during the C-OPTIMISE study compared to those who remained on CZP. These findings confirm that patients with axSpA who achieve sustained remission benefit from continued CZP treatment, either with the full or reduced maintenance dose, over treatment withdrawal.
REFERENCES:
[1]Landewé R. Ann Rheum Dis 2020;79:920–8.
Acknowledgements: This study was funded by UCB Pharma. Editorial services were provided by Costello Medical.
Disclosure of Interests: Lianne S. Gensler Consultant of: AbbVie, Eli Lilly, Gilead, GSK, Novartis, Pfizer, UCB Pharma, Grant/research support from: Pfizer, Xenofon Baraliakos Speakers bureau: AbbVie, BMS, Chugai, Eli Lilly, Galapagos, Gilead, Novartis, Merck, Pfizer, UCB Pharma, Paid instructor for: AbbVie, BMS, Chugai, Eli Lilly, Galapagos, Gilead, Novartis, Merck, Pfizer, UCB Pharma, Consultant of: AbbVie, BMS, Chugai, Eli Lilly, Galapagos, Gilead, Novartis, Merck, Pfizer, UCB Pharma, Grant/research support from: AbbVie, Merck, Novartis, Lars Bauer Shareholder of: UCB Pharma, Employee of: UCB Pharma, Bengt Hoepken Shareholder of: UCB Pharma, Employee of: UCB Pharma, Thomas Kumke Shareholder of: UCB Pharma, Employee of: UCB Pharma, Mindy Kim Shareholder of: UCB Pharma, Employee of: UCB Pharma, Robert B.M. Landewé Speakers bureau: Abbott, Amgen, BMS, Centocor, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth, Consultant of: Abbott, Ablynx, Amgen, AstraZeneca, BMS, Centocor, GSK, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth, Grant/research support from: Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth