

Background: Behçet’s syndrome, a chronic, multi-system variable vessel vasculitis, is often characterized by painful oral ulcers (OU) affecting quality of life (QoL). Apremilast (APR), an oral PDE4 inhibitor, demonstrated efficacy in OU treatment in the phase 3 multinational RELIEF study.
Objectives: To evaluate APR efficacy in OU treatment in patients with active Behçet’s syndrome in a prespecified subgroup of patients enrolled in 13 European RELIEF sites (France, Germany, Greece, and Italy).
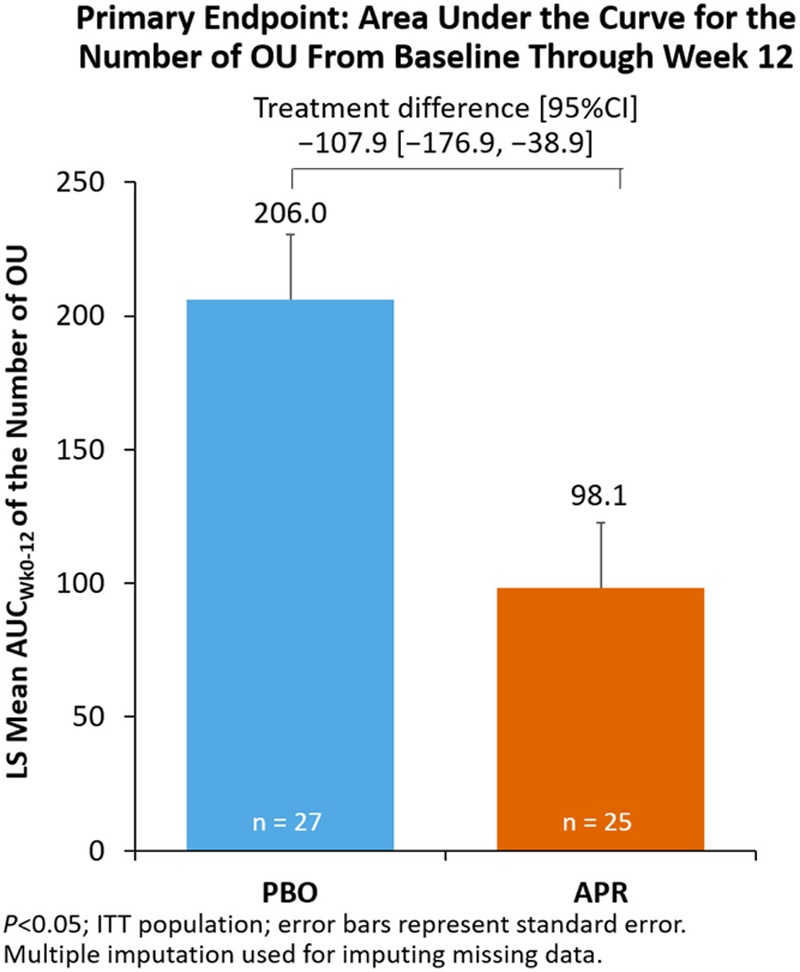
Methods: patients were adults with active Behçet’s syndrome and ≥3 OU at randomization or ≥2 OU at screening and randomization, without active major organ involvement. Patients were randomized (1:1) to APR 30 mg BID or PBO during a 12-week double-blind phase. The primary endpoint was area under the curve for the number of OU through Week 12 (AUC Wk0-12 ). Other outcomes were OU pain visual analog scale (VAS); achievement of OU complete response (ie, OU-free) and maintenance of OU complete response (ie, complete response at Week 6 and remaining OU-free for ≥6 additional weeks); OU partial response (ie, OU reduction ≥50%); disease activity (Behçet’s Syndrome Activity Score [BSAS]; Behçet’s Disease Current Activity Form [BDCAF], including Behçet’s Disease Current Activity Index [BDCAI], and Patient’s and Clinician’s Perception of Disease Activity); and QoL (BDQoL; Short Form Health Survey version 2 [SF-36v2], including Physical Functioning [PF] scale and Physical and Mental Component Summary [PCS, MCS]).
Results: Of 207 patients randomized and treated in RELIEF, 52 were in the European subgroup. Mean (±SD) age in the subgroup was 39 (±12) years; 54% were women. Baseline disease characteristics were similar between treatment groups (
Conclusion: In the European subgroup of patients with Behçet’s syndrome and OU in RELIEF, APR resulted in greater reduction in OU count, OU pain, and disease activity as well as favorable treatment effect on QoL measures than PBO. These results are consistent with the efficacy of APR treatment in the overall RELIEF population.
| Baseline Disease Characteristics, Mean* | PBO (n = 27) | APR (n = 25) | |
| Duration of BD, years | 9.0 | 8.2 | |
| OU count | 3.8 | 4.0 | |
| OU pain (VAS 0-100) | 60.6 | 64.2 | |
| BSAS (0-100) | 38.7 | 41.4 | |
| BDCAI (0-12) | 3.5 | 3.6 | |
| BDQoL (0-30) | 10.5 | 9.0 | |
| Efficacy Outcomes at 12 Weeks* | PBO (n = 27) | APR (n = 25) | Treatment Difference [95% CI] |
| OU pain (VAS 0-100), mean † | –17.7 | –48.7 | –31.0 [–44.7, –17.3] |
| OU complete response, n (%) ‡ | 4 (14.8) | 16 (64.0) | 51.5 [29.8, 73.3] |
| OU maintained response, n (%) ‡ | 1 (3.7) | 8 (32.0) | 26.7 [7.4, 46.0] |
| OU partial response, n (%) ‡ | 11 (40.7) | 21 (84.0) | 46.0 [23.9, 68.0] |
| BSAS (0-100) †,§ | –5.23 | –20.68 | –15.5 [–22.6, –8.3] |
| BDCAI (0-12) †,§ | –0.0 | –1.4 | –1.4 [–2.2, –0.6] |
| Patient’s Perception of Disease Activity †,§ | –0.4 | –1.6 | –1.2 [–2.1, –0.4] |
| Clinician’s Overall Perception of Disease Activity †,§ | −0.6 | −1.7 | –1.0 [–1.7, –0.4] |
| BDQoL (0-30) †,§ | –1.25 | –2.37 | –1.12 [–3.8, 1.5] |
| SF-36v2 MCS (0-100) †,§ | –2.1 | 4.2 | 6.3 [2.2, 10.4] |
*ITT population. † LS mean of the change from baseline at Week 12. ‡ Non-responder imputation for missing data. § LOCF approach. All efficacy endpoints (except BDQoL) were significant at the level of P <0.05.
Acknowledgements : This study was funded by Celgene. Additional analyses were funded by Amgen Inc. Writing support was funded by Amgen Inc. and provided by Kristin Carlin, RPh, MBA, of Peloton Advantage, LLC, an OPEN Health company.
Disclosure of Interests: Alfred Mahr Speakers bureau: Chugai; Roche, Consultant of: Celgene; Chugai, Gulen Hatemi Speakers bureau: AbbVie, Novartis, and UCB, Grant/research support from: Celgene, Mitsuhiro Takeno Speakers bureau: AbbVie, Esai, and Mitsubishi-Tanabe, Consultant of: Celgene, Grant/research support from: Novartis, Doyoung Kim: None declared, Melike Melikoglu: None declared, david Saadoun Consultant of: AbbVie, Celgene, Janssen, and Roche, Grant/research support from: AbbVie and Roche, Christos C. Zouboulis Speakers bureau: Amgen, Galderma, Pierre Fabre, PPM and Sobi, Consultant of: AbbVie, AccureAcne, Almirall, Bayer Healthcare, GSK/Stiefel, Incyte, Inflarx, Janssen, Novartis, PPM, Regeneron, and UCB, Grant/research support from: Celgene, NAOS-BIODERMA, and Relaxera, Sue Cheng Employee of: Amgen Inc, Sven Richter Employee of: Amgen Inc, Shauna Jardon Employee of: Amgen Inc, Maria Paris Employee of: Amgen Inc, Mindy Chen Employee of: Amgen Inc, Yusuf Yazici Consultant of: Bristol-Myers Squibb, Celgene, Genentech, and Sanofi