

Background: Interstitial lung disease (ILD) is a severe complication of RA. Abatacept (ABA) have demonstrated efficacy in RA-ILD [1,2], although combined treatment with MTX or others DMARDs remain controversial.
Objectives: To assess the efficacy and safety of ABA in monotherapy (ABA MONO ) versus combined-ABA, ABA plus MTX(ABA MTX ) or ABA plus other non-MTX DMARDs (ABA NON-MTX ), in RA-ILD.
Methods: Observational multicenter study of RA-ILD caucasian patients treated with ABA. We analyzed in three groups (ABA MONO , ABA MTX , ABA NON-MTX ) the following outcomes: a ) Dyspnea, b ) FVC and DLCO, c ) HRCT, d ) DAS28-ESR, e ) corticosteroid-sparing effect. Differences between basal and final follow-up were evaluated. Multivariable linear regression was used between the three groups.
Results: We studied 263 RA-ILD patients (mean age 64.6±10 years) [ABA
MONO
(n=111), ABA
MTX
(n=46) and ABA
NON-MTX
(n=106)]. At baseline, ABA
MONO
patients were older (67±10 years) and took higher prednisone dose (10 [IQR 5-15] mg/day). There was no statistically significant differences in sex, seropositivity, ILD patterns, FVC, DLCO or disease duration. In all groups, most patients experienced stabilization or improvement in FVC, DLCO, dyspnea, HRCT as well as improvement in DAS28-ESR. A statistically significant difference between basal and final follow-up was only found in corticosteroid-sparing effect in ABA
MTX
or ABA
NON-MTX
(
Effect in FVC, DLCO, dyspnea (mMRC) and HRCT pulmonary scan after abatacept.
|
ABA
MONO
|
ABA
MTX
|
ABA
NON-MTX
| ABA MTX vs ABA MONO | ABA NON-MTX vs ABA MONO | |||||||
| p | p | p | p* | Unadjusted | Adjusted** | Unadjusted | Adjusted** | ||||
| Follow-up, median [IQR] months | 12 [6-36] | 12 [6-36] | 18[12-36] | 0.40 | 0.67 | 0.17 | |||||
| Differences between basal and final follow-up | |||||||||||
| FVC, % | -0.5 (-2.5, 1.5) | 0.64 | 1.2(-0.6, 3.1) | 0.17 | -1.2 (-2.9, 0.5) | 0.17 | 0.33 | 0.30 | 0.39 | 0.59 | 0.90 |
| DLCO, % | 1.8 (-0.7, 4.34) | 0.16 | 0.5 (-3.8, 4.8) | 0.82 | -1.5 (-4.1, 1.1) | 0.26 | 0.20 | 0.58 | 0.80 | 0.07 | 0.32 |
| mMRC, n (%) | |||||||||||
| Worsening | 5 (5) | 3 (8) | 5 (5) | 0.83 | 0.47 | 0.99 | |||||
| Stable or improving | 93 (95) | 36 (92) | 87 (95) | ||||||||
| HRCT pulmonary scan, n (%) | |||||||||||
| Worsening | 13 (28) | 2 (11) | 15 (25) | 0.24 | 0.10 | 0.78 | |||||
| Stable or improving | 34 (72) | 19 (89) | 44 (75) | ||||||||
| DAS28-ESR | -1.5 (-1.9, -1.0 ) | 0.000 | -1.2 (-1.8, -0.6 ) | 0.000 | -1.5 (-1.8, -1.2 ) | 0.000 | 0.74 | 0.58 | 0.92 | ||
| Prednisone, mg/day | -3.8 (-8.3, 0.8) | 0.10 | -2.7 (-4.6, -0.8 ) | 0.006 | -4.8 (-6.3, -3.4 ) | 0.000 | 0.69 | 0.67 | 0.65 | ||
Differences in DAS28-ESR, prednisone, FVC and DLCO are expressed as mean difference (95%CI) comparing final follow-up minus basal values.
*Differences between the 3 groups.
**Differences between ABA MTX vs. ABA MONO , and between ABA NON-MTX vs ABA MONO are adjusted for age, disease duration until abatacept treatment, and DAS28 and prednisone dose at baseline.
Abbreviations (DAS28-ESR: Disease activity score-erythrocyte sedimentation rate; DLCO: Carbon Monoxide Diffusing Capacity; HRCT: High resolution computed tomography; FVC: Forced vital capacity, mMRC: modified Medical Research Council scale
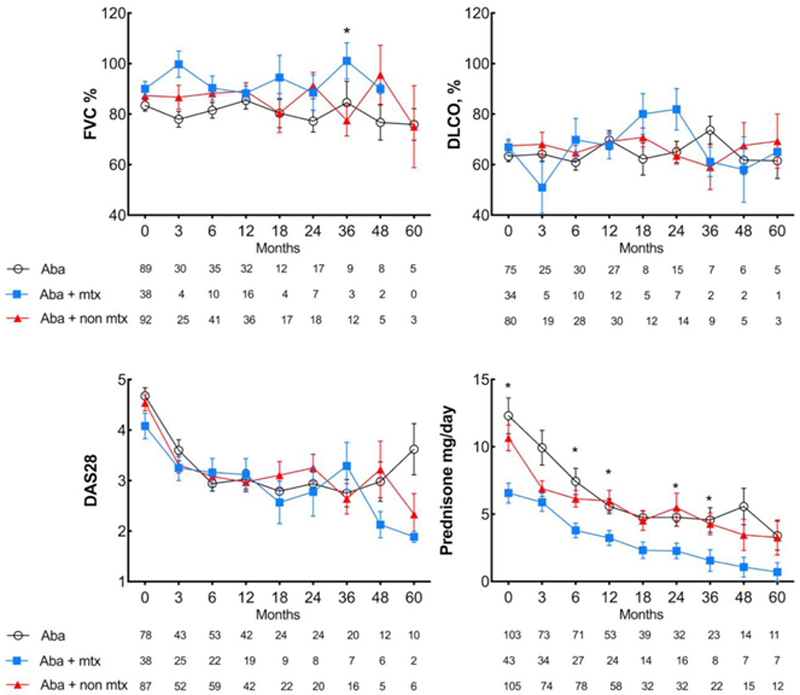
Conclusion: In caucasian individuals with RA-ILD, ABA MONO or ABA MTX or ABA NON-MTX seems to be equally effective and safe. However, a corticosteroid-sparing effect is only observed in combined-ABA.
REFERENCES:
[1]Fernández-Díaz C, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: A national multicenter study of 63 patients. Semin Arthritis Rheum. 2018 Aug;48(1):22-27. doi: 10.1016/j.semarthrit.2017.12.012.
[2]Fernández-Díaz C, et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatology (Oxford). 2020 Dec 1;59(12):3906-3916. doi: 10.1093/rheumatology/keaa621.
Acknowledgements: Spanish Collaborative Group of Interstitial Lung Disease Associated to Rheumatoid Arthritis
Disclosure of Interests: Carlos Fernández-Díaz Speakers bureau: Roche, bristol myers squibb, Belén Atienza-Mateo: None declared, Santos Castañeda: None declared, Rafael Melero: None declared, Francisco Ortiz-Sanjuán: None declared, Ivette Casafont-Solé: None declared, J. Loricera: None declared, Sebastián C Rodriguez-García: None declared, Iván Ferraz-Amaro: None declared, Miguel A González-Gay: None declared, Ricardo Blanco Speakers bureau: bristol myers squibb