

Background: Filgotinib (FIL), an oral janus kinase 1 (JAK1) inhibitor, has been evaluated in three phase 3 clinical studies (FINCH 1-3) in adults with moderately-to-severely active rheumatoid arthritis (RA). Patients with RA who currently smoke (a predisposing factor for RA) have been reported to be less likely to respond to anti-TNFα treatment and more likely to discontinue or switch treatment. 1,2,3 However, the impact of smoking on JAKi efficacy in RA patients is unknown.
Objectives: A post-hoc sub-group analysis of FINCH patient (pt) data was performed in order to identify associations with smoking status.
Methods: Data from 3452 RA pts participating in the FINCH3 (MTX-naïve; NCT02886728), FINCH1 (MTX-IR; NCT02889796), or FINCH2 (bDMARD-IR; NCT02873936) clinical trials were included for analysis of clinical response at weeks 12 and 24. Logistic regression models were fitted to assess the effects of smoking status on categorical clinical endpoints (ACR20/50/70, CDAI ≤ 10, DAS28(CRP) ≤3.2 or < 2.6). Adjustments were made for covariates selected based on previously published work 1 and listed in the Figure 1 caption. No adjustments of p-values were made for multiple testing.
Results: In the MTX-IR population (FINCH1), current (12% of enrolled patients) and former (13%) smokers treated with ADA+MTX had a lower week 12 ACR50 response rate compared to non-smokers (25% and 28% vs. 39%, nominal p = 0.095 and p=0.21, respectively). In contrast, the ACR 50 response at week 12 in FIL+MTX treated pts showed no association with smoking status. Former smokers had higher response rates than non-smokers in the MTX-IR (FINCH1) and MTX naïve (FINCH3) populations (
Difference in adjusted ACR50 response rate compared to non-smokers for FINCH1/2/3.
| PBO+MTX ‡ | FIL100+MTX | FIL200+MTX | ||
| FINCH1 | Current | -1% | +2.4% | +3.3% |
| Former | -6% | +12% | +16% (*) | |
| FINCH2 † | Current | 0% | +13% | -9% |
| Former | +9% | -7% | +4% | |
| FINCH3 | Current | 0% | +1% | +4% |
| Former | +4% | +20% (*) | +17% (*) | |
ACR50 response rate based on fully adjusted logistic regression model. † : FINCH2 patients received either MTX or csDMARD. ‡ FINCH3 had active comparator arm of MTX together with PBO to maintain blind. *: p < 0.05, **: p < 0.01, ***: p < 0.001
FINCH1 adjusted Week 12 ACR50 response rate stratified by smoking status. ACR50 response rate based on fully adjusted logistic regression model. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Former and current smokers that received FIL200mg + MTX showed a higher response rate compared to similar ADA+MTX patients.
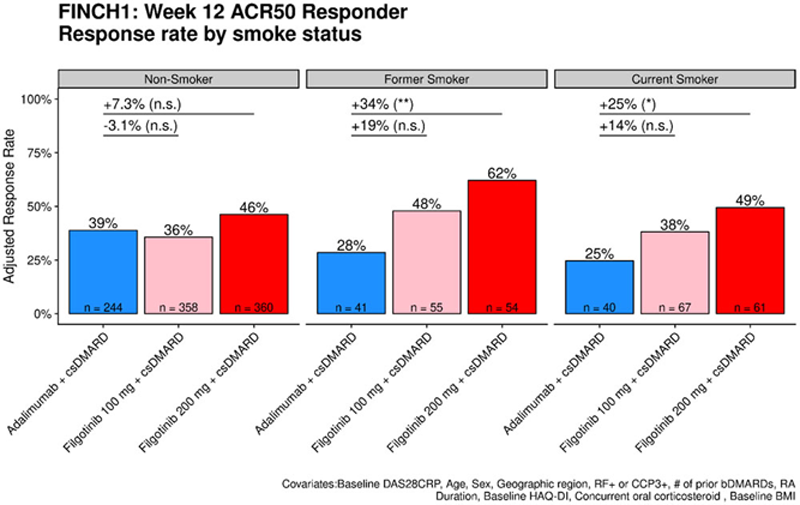
Conclusion: This exploratory analysis showed that current and former smokers with RA who received ADA+MTX trended toward a lower response rate compared to non-smokers. In contrast, FIL+MTX was found to be similarly efficacious independent of smoking status within both the MTX-IR and MTX-naïve RA populations. Current or former smokers were more likely to respond to FIL200mg + MTX compared to ADA+MTX across a range of endpoints. Given the small number of current and former smokers enrolled in these studies, further studies of the efficacy of JAK inhibitors and the mechanism of reduced response to anti-TNFs in patients with a history of smoking are warranted.
REFERENCES:
[1]Hyrich KL. Rheumatology 2006;45:1558–1565; 2. Canhao H. Rheumatology 2012;51:2020–2026; 3. McCulley CB. Clin Exp Rheumatol 2019;28(37):422–428
Disclosure of Interests: Paul Emery Consultant of: Pfizer, Abbvie, Amgen, MSD, Roche, Sanofi, BMS, Novartis, Lilly, Gilead, Samsung, Celltrion, Grant/research support from: Abbvie, BMS, Samsung, Bryan Downie Shareholder of: Gilead, Employee of: Gilead, Jinfeng Liu Shareholder of: Gilead, Roche, Employee of: Gilead, Rachael Hawtin Shareholder of: Gilead, Employee of: Gilead, Jeffrey R. Curtis Consultant of: Abbvie, Amgen, BMS, Corrona, Eli Lilly, Jannsen, Myriad, Pfizer, Regeneron, Roche, and UCB, Grant/research support from: Abbvie, Amgen, BMS, Corrona, Eli Lilly, Jannsen, Myriad, Pfizer, Regeneron, Roche, and UCB, Gerd Rüdiger Burmester Speakers bureau: Abbvie, Gilead, Lilly, Pfizer, Consultant of: Abbvie, Gilead, Lilly, Pfizer