

Background: Biosimilar products of biological disease-modifying antirheumatic drugs (bDMARDs) entered the Swedish market in 2015, with regulatory approvals based on head to head trials of limited duration. Longer-term comparative drug survival, in clinical practice, remains less well documented.
Objectives: To compare survival on drug between biosimilars and their originator products among first starters of etanercept, infliximab, adalimumab and rituximab.
Methods: Data from the Swedish Rheumatology Quality register (SRQ) was used to identify and follow patients who started a first ever treatment with etanercept since April 2015 (originator=ETA,biosimilar= SB4), infliximab since March 2014 (originator=IFX,biosimilar= CT-P13), adalimumab since January 2018 (originator=ADA biosimilars=SB5, ABP501), or rituximab since January 2018 (originator=RIT,biosimilar= GP2013), through December 31 st , 2019, date of first discontinuation of the drug, or death. Discontinuation was defined as lack of effectiveness or adverse events, while other reasons for interruption of the drug (including non-medical switch) were considered censoring events. Descriptive characteristics were collected from the SRQ and tabulated. Hazard ratios (HR) of discontinuation were estimated using Cox regression, with each drug analyzed separately, adjusted for age,sex,indication,line of treatment,disease duration,year of treatment start,region and concomitant use of csDMARD.
Results: 9274 patients started etanercept(49% SB4), 3609 started infliximab(64% CT-P13), 3117 started adalimumab(27% SB5, 14% ABP 501), and 763 started rituximab(39% GP2013),
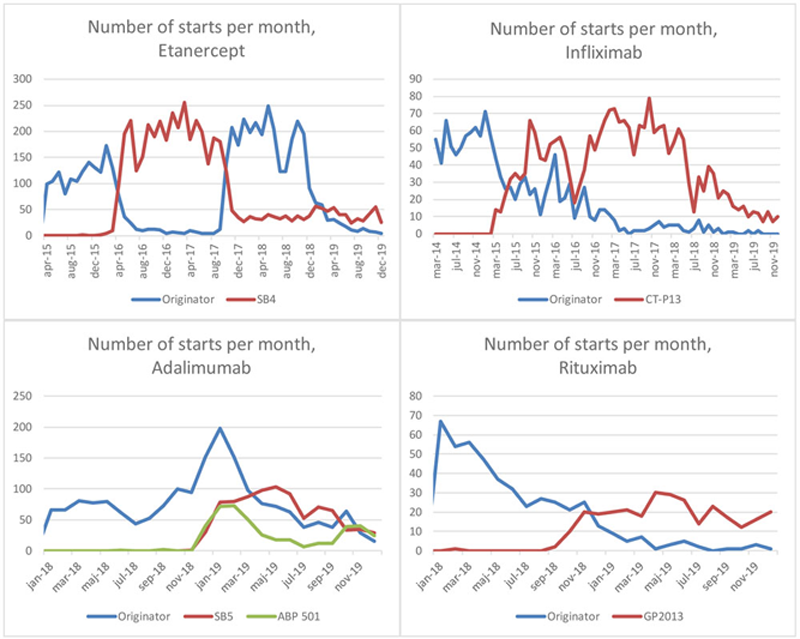
The introduction of a biosimilar was typically followed by a decrease in the uptake of the originator, but for ETA a change in pricing in 2018 later led to a reversal of this pattern (
For IFX,ADA,and RIT, survival on drug was similar for the originator and its biosimilar(s). For ETA,risk of discontinuation was somewhat lower for the biosimilar than for the originator(adjusted HR:0.87,95% confidence interval:0.79-0.95),
Hazard ratios of discontinuation and descriptive characteristics of biosimilar vs. originator among first starters of each molecule, until 31 st December 2019.
| Etanercept | Infliximab | Adalimumab | Rituximab | ||||||
| Originator | SB4 | Originator | CT-P13 | Originator | SB5 | ABP 501 | Originator | GP2013 | |
| N | 4721 | 4553 | 1308 | 2301 | 1834 | 852 | 431 | 465 | 298 |
| Discontinuation | 1289 | 1236 | 582 | 878 | 399 | 139 | 80 | 57 | 26 |
| Adjusted hazard ratios* | Ref | 0.87 (0.79-0.95) | Ref | 1.14 (0.99-1.31) | Ref | 1.02 (0.83-1.26) | 1.16 (0.88-1.52) | Ref | 1.12 (0.68-1.85) |
| Age, mean years (std ) | 51 (16) | 51 (15) | 49 (16) | 49 (16) | 48 (15) | 52 (15) | 51 (15) | 59 (15) | 60 (15) |
| Female, % | 67% | 65% | 61% | 64% | 62% | 64% | 65% | 75% | 76% |
| RA, % | 46% | 48% | 39% | 35% | 33% | 42% | 43% | 61% | 76% |
| Bionaïve, % | 72% | 72% | 76% | 69% | 45% | 52% | 43% | 53% | 38% |
| Disease duration, mean years (std ) | 11 (12) | 11 (11) | 11 (11) | 11 (11) | 12 (13) | 12 (11) | 14 (15) | 14 (19) | 15 (11) |
| DAS28, mean | 4.0 (1.3) | 4.0 (1.4) | 4.1 (1.4) | 4.1 (1.4) | 3.7 (1.4) | 3.8 (1.3) | 4.0 (1.3) | 4.5 (1.4) | 4.7 (1.4) |
| Concomitant csDMARDs, % | 45% | 47% | 57% | 48% | 37% | 49% | 42% | 36% | 43% |
Abbreviations: RA=rheumatoid arthritis. csDMARDs=conventional synthetic DMARD, std=standard deviation.
Number of starts of biosimilars compared to the originator during the follow-up time, by molecule
Conclusion: Despite their identical indications and therapeutic positioning, there are some differences in the baseline characteristics between patients who start ADA, IFX and RIT and their biosimilars. There are no differences in drug survival between originator and biosimilar with the possible exception of etanercept although the observed difference should be interpreted in light of possible unmeasured or residual channeling.
Disclosure of Interests: Daniela Di Giuseppe: None declared, Hannah Bower: None declared, Bénédicte Delcoigne: None declared, Thomas Frisell: None declared, Katerina Chatzidionysiou Consultant of: Eli Lilly, AbbVie and Pfizer, Ulf Lindström: None declared, Christopher Sjowall: None declared, Elisabet Lindqvist: None declared, Johan Askling Grant/research support from: Abbvie, Astra-Zeneca, BMS, Eli Lilly, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB,