

Background: Biosimilar drugs follow a tailored approval pathway that usually includes a Phase III comparative efficacy randomized controlled trial with a high internal but low external validity. Therefore, observational studies with high external validity are important to reassure patients and physicians that there are no clinically meaningful differences in effectiveness between a biosimilar and its reference drug. A EULAR Task Force systematic review and others have noted that recent comparative effectiveness studies often do not disclose applied analytical methods in sufficient detail, with many studies not adjusting for confounders nor accounting for attrition or missing data. 1,2
Objectives: To apply the EULAR Points to Consider for Comparative Effectiveness Research (CER) in an analysis of reference etanercept (ETN) and SB4 biosimilar ETN in patients with rheumatoid arthritis (RA) treated in ordinary clinical practice in Norway.
Methods: ETN-naÏve patients with RA starting ETN treatment between January 2010 and July 2018 at five centres in Norway were followed for at least 1 year; the 2 cohorts remained on either ETN or SB4 throughout. The primary outcome was DAS28 at Week 52. This CER has been designed to formally assess equivalence for DAS28, based on the accepted equivalence margin of 0.6. 3 Conventional regression and propensity score (PS) models have been applied for the primary outcome evaluation of DAS28 at Week 52. Based on clinical knowledge, the confounders adjusted for in the step-wise PS model were age, gender, DAS28, order of biologics, and concomitant conventional synthetic disease-modifying anti-rheumatic drugs. A standardized difference (d) of <0.1 indicates a good match.
Results: In the unmatched sample, there were 575 patients treated with reference ETN and 299 treated with SB4. Before PS matching, baseline mean (SD) DAS28 was different between the ETN and SB4 groups, 4.3 (1.2) vs 4.0 (1.3), (d) = 0.25. After PS matching, there were 176 RA patients in each group; baseline mean (SD) DAS28 was 4.1 (1.2) vs 4.1 (1.3), (d) = 0.05. At Week 52, the difference (mean [95% confidence interval (CI)]) between reference ETN and SB4 for primary outcome DAS28 at Week 52 was -0.02 (-0.33 to 0.29) in the unmatched analysis. Since the entire 95% CI is within the pre-defined equivalence margin of 0.6, equivalence at Week 52 has formally been shown. The analysis of the PS matched groups to Week 52 is ongoing and results will be presented in the poster.
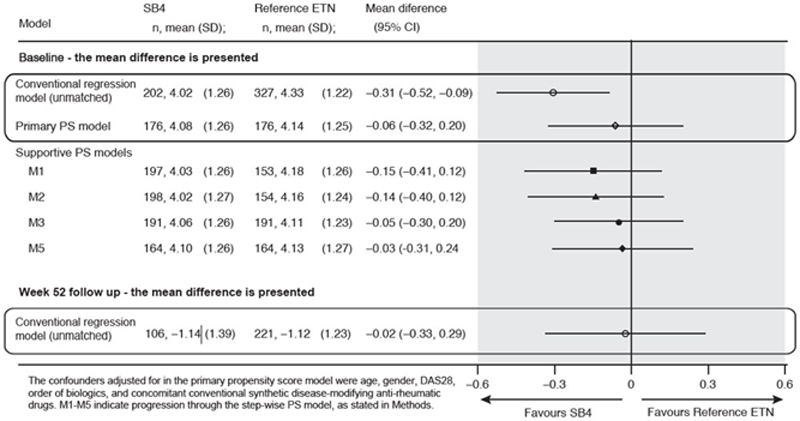
Conclusion: Our results show the importance of adopting proper analytical techniques when comparing a biosimilar with its reference product. A conventional regression model may not fully account for differences in key clinical measures (in this instance, disease activity) between the two groups at baseline, and therefore the Week 52 results might be biased. The propensity score matched model ensures comparability of the groups at baseline and therefore the validity of the Week 52 results should be more robust.
REFERENCES:
[1]Cantini F and Benucci M. Mandatory, cost-driven switching from originator etanercept to its biosimilar SB4: possible fallout on non-medical switching. Ann Rheum Dis 2020; 79: e13.
[2]Lauper K KJ, De Wit M, Fautrel B, et al. A Systematic Review to Inform the EULRA Points to Consider When Analysing and Reporting Comparative Effectiveness Research With Observational Data in Rheumatology. Annals of the Rheumatic Diseases 2020; 79:
[3]Fransen J and van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005; 23: S93-99.
Acknowledgements: The authors wish to acknowledge Janet Addison and Ulrich Freudensprung of Biogen for their intellectual contributions to this abstract and Bjørg Tilde Fevang for providing data from Haukeland University Hospital in Bergen. Editorial support for the preparation of this abstract was provided by Excel Scientific Solutions (Fairfield, CT, USA); funding was provided by Biogen International GmbH.
Disclosure of Interests: Glenn Haugeberg Grant/research support from: Biogen, Gunnstein Bakland: None declared, Erik Rødevand: None declared, Inger Johanne Widding Hansen: None declared, Andreas Diamantopoulos: None declared, Are Hugo Pripp: None declared