

Background: Differences in efficacy outcomes favouring males vs females with rheumatoid arthritis (RA) have been reported with conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) and tumour necrosis factor inhibitors; results with Janus kinase inhibitors are less clear.
Objectives: To assess the impact of sex on efficacy, safety and persistence in tofacitinib RA clinical trials.
Methods: Efficacy and safety analyses included data pooled from Phase (P)3 randomised controlled trials (RCTs) of patients (pts) with RA and an inadequate response (IR) to methotrexate (NCT00847613; NCT00853385) or ≥1 DMARD (NCT00856544) who received tofacitinib 5 or 10 mg twice daily (BID), adalimumab (ADA) 40 mg Q2W or placebo (PBO), with background csDMARDs. Persistence analyses of pts receiving tofacitinib 5 or 10 mg BID ± csDMARDs used data pooled from two long-term extension trials (NCT00661661; NCT00413699). Efficacy outcomes to Month (M)12 included: ACR20/50/70 responses, changes from baseline (Δ; BL) in DAS28-4(ESR), CDAI, HAQ-DI and FACIT-F, and DAS28-4(ESR) remission (<2.6). Safety was evaluated to M24 for tofacitinib and ADA. Kaplan-Meier persistence analysis estimated time to discontinuation.
Results: 2265 pts were included from P3 RCTs. Demographics and BL characteristics were comparable across sexes and treatments. Tofacitinib or ADA vs PBO generally led to significantly higher ACR20/50/70 responses in both sexes through M6. To M12, ACR20/50/70 responses were broadly comparable across active treatments and between sexes, with significant differences favouring males at some time points, including M3 (
Safety summary to M24 in pooled DMARD-IR P3 RCTs
|
Tofacitinib
|
Tofacitinib
| ADA | ||||
|
Pts with events,
|
Females
|
Males
|
Females
|
Males
|
Females
|
Males
|
| AEs | 562 (79.5) | 85 (63.9) | 529 (75.8) | 107 (78.1) | 119 (73.5) | 30 (71.4) |
| SAEs | 107 (15.1) | 17 (12.8) | 71 (10.2) | 24 (17.5) | 13 (8.0) | 6 (14.3) |
| Severe AEs | 86 (12.2) | 12 (9.0) | 55 (7.9) | 22 (16.1) | 14 (8.6) | 5 (11.9) |
| Discontinuations due to AEs | 87 (12.3) | 10 (7.5) | 88 (12.6) | 10 (7.3) | 17 (10.5) | 5 (11.9) |
| Death | 6 (0.8) | 4 (3.0) | 0 | 3 (2.2) | 1 (0.6) | 2 (4.8) |
| AESI | ||||||
| Serious infections | 28 (4.0) | 6 (4.5) | 27 (3.9) | 6 (4.4) | 2 (1.2) | 1 (2.4) |
| All HZ (non-serious/serious) | 35 (5.0) | 7 (5.3) | 43 (6.2) | 5 (3.6) | 2 (1.2) | 3 (7.1) |
| MACE | 5 (0.7) | 0 | 2 (0.3) | 3 (2.2) | 0 | 3 (7.1) |
| Malignancies (excl. NMSC) | 7 (1.0) | 1 (0.8) | 9 (1.3) | 1 (0.7) | 0 | 1(2.4) |
| NMSC | 2 (0.3) | 5 (3.8) | 4 (0.6) | 2 (1.5) | 1 (0.6) | 1 (2.4) |
| Venous thromboembolism | 3 (0.4) | 0 | 3 (0.4) | 1 (0.7) | 0 | 0 |
HZ, herpes zoster; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer
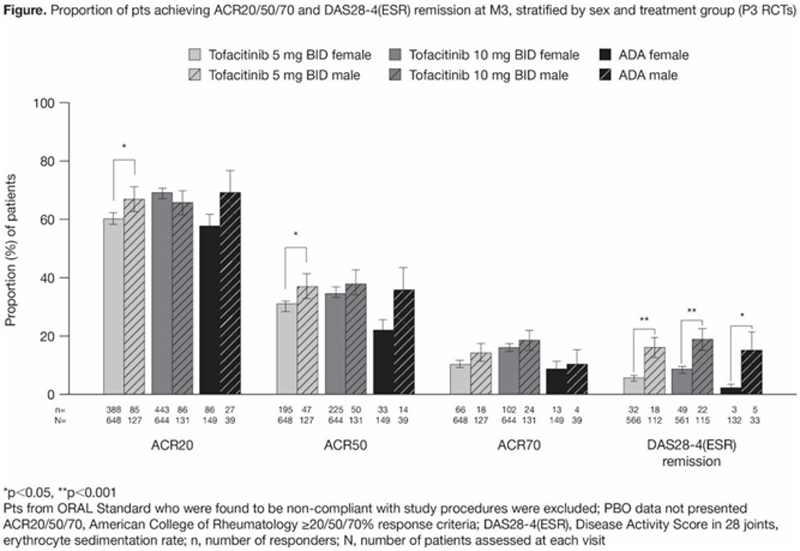
Conclusion: Efficacy outcomes with tofacitinib and ADA were generally higher in males and comparable in females vs previously reported mixed population response rates for advanced therapies. Safety findings did not reveal a consistent pattern between sexes. Tofacitinib persistence was similar between sexes.
Acknowledgements: Study sponsored by Pfizer Inc. Medical writing support was provided by Christina Viegelmann, CMC Connect, and funded by Pfizer Inc.
Disclosure of Interests: H Niall Jones Consultant of: Pfizer Inc, Vibeke Strand Consultant of: AbbVie, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Corrona, Eli Lilly, Galapagos, Genentech/Roche, Gilead, GlaxoSmithKline, Ichnos, Inmedix, Janssen, Kiniksa, Merck, Myriad Genetics, Novartis, Pfizer Inc, Regeneron, Samsung, Sandoz, Sanofi, Scipher, SetPoint Medical, UCB, Hendrik Schulze-Koops Consultant of: AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead Sciences, Hexal Sandoz, Hospira, Janssen-Cilag, MSD, Novartis, Pfizer Inc, Roche, UCB, Grant/research support from: Novartis, Pfizer Inc, Eduardo Mysler Speakers bureau: AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer Inc, Roche, Sanofi, Grant/research support from: Eli Lilly, Pfizer Inc, Roche, Cassandra Kinch Shareholder of: Pfizer Canada ULC, Employee of: Pfizer Canada ULC, David C Gruben Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Rebecca Germino Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Carol A. Connell Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Lihi Eder Speakers bureau: AbbVie, UCB, Consultant of: AbbVie, Celgene, Eli Lilly, Novartis, Pfizer Inc, UCB, Grant/research support from: AbbVie, Eli Lilly, Pfizer Inc, UCB