

Background: The Janus kinase-1 preferential inhibitor filgotinib (FIL) improved signs and symptoms of rheumatoid arthritis (RA) across the FIL clinical program. 1–3
Objectives: To assess FIL efficacy across geographic regions.
Methods: Pooled data from patients (pts) meeting 2010 ACR/EULAR RA criteria randomised to once-daily FIL 200 mg (FIL200), FIL100 mg (FIL100), or placebo (PBO) with background conventional synthetic disease-modifying antirheumatic drugs in DARWIN 1 (P2; up to week [W]12) and FINCH 1–2 (P3; up to W24) studies were evaluated. Data were analysed by region: North America, South and Central America, Western Europe, Eastern Europe, Asia, South East (SE) Asia, and Other. W12 American College of Rheumatology 20% improvement (ACR20) and W24 Disease Activity Score in 28 joints (C-reactive protein) (DAS28[CRP]) <2.6 and ≤3.2 response rates were analysed by a logistic regression model. Change from baseline (CFB) in Health Assessment Questionnaire-Disability Index (HAQ-DI) at W12 was analysed by a mixed-effects model for repeated measures. Analyses were exploratory and not adjusted for multiplicity.
Results: Despite high PBO response rates in Eastern Europe and South and Central America, greater proportions of pts receiving FIL200 or FIL100 vs PBO achieved ACR20 at W12 (
P
<0.05) in all regions, except Other (with lowest sample size, n = 69), where both FIL doses were numerically greater than PBO (
Proportion of pts achieving ACR20 and LSM change from baseline HAQ-DI at week 1
| ACR20 | HAQ-DI | |||||
| FIL200 | FIL100 | FIL200 | FIL100 | FIL200 | FIL100 | |
| North America | 64.8 * | 58.3 * | 33.8 | −0.63 * | −0.58 * | −0.34 |
| n = 455 | (56.7, 72.9) | (50.3, 66.4) | (26.0, 41.6) | (−0.70, −0.56) | (−0.65, −0.51) | (−0.41, −0.27) |
| South and Central America | 77.2 | 77.3 | 57.4 | −0.77 * | −0.67 * | −0.43 |
| n = 283 | (68.1, 86.3) † | (65.8, 86.2) † | (46.9, 68.0) | (−0.85, −0.68) | (−0.75, −0.59) | (−0.52, −0.35) |
| Western Europe | 69.4 * | 68.3 * | 24.4 | −0.69 * | −0.61 * | −0.28 |
| n = 135 | (55.5, 83.3) | (52.8, 83.8) | (10.8. 38.1) | (−0.80, −0.58) | (−0.73, −0.49) | (−0.40, −0.17) |
| Eastern Europe | 77.1 * | 69.1 * | 54.6 | −0.62 * | −0.51 * | −0.34 |
| n = 822 | (71.9, 82.3) | (63.5, 74.7) | (48.5, 60.7) | (−0.68, −0.56) | (−0.57, −0.45) | (−0.40, −0.28) |
| Asia | 81.0 * | 60.0 † | 37.7 | −0.83 * | −0.61 † | −0.42 |
| n = 236 | (71.7, 90.3) | (48.6, 71.4) | (26.2, 49.1) | (−0.92, −0.73) | (−0.70, −0.52) | (−0.52, −0.33) |
| South East Asia | 70.2 † | 71.1 † | 39.5 | −0.61 | −0.57 | −0.45 |
| n = 135 | (56.1, 84.4) | (56.8, 85.5) | (23.8, 55.3) | (−0.73, −0.49) | (−0.69, −0.45) | (−0.58, −0.33) |
| Other | 60.0 | 52.4 | 39.1 | −0.56 ‡ | −0.60 ‡ | −0.33 |
| n = 69 | (38.8, 81.2) | (28.6, 76.1) | (17.0, 61.2) | (−0.72, −41) | (−0.76, −0.43) | (−0.49, −0.17) |
| Overall | 73.4 * | 66.4 * | 45.3 | −0.71 * | −0.61 * | −0.40 |
| N = 2135 | (70.1, 76.8) | (62.9, 70.0) | (41.5, 49.0) | (−0.76, −0.66) | (−0.66, −0.56) | (−0.45, −0.35) |
Includes only patients initially randomised to the treatment groups in each study for the comparison of interest. ACR20 presented as percentage (95% CI); 95% CI was based on normal approximation method with a continuity correction; P values calculated from the logistic regression.HAQ-DI presented as LSM (95% CI); LSM, 95% CI, and P value calculated from a mixed-effects model for repeated measures.*P <0.001, †P <0.01, ‡P <0.05; not adjusted for multiplicity.
ACR20, American College of Rheumatology 20% improvement; CI, confidence interval; FIL, filgotinib; HAQ-DI, Health Assessment Questionnaire-Disability Index; LSM, least square mean; PBO, placebo.
At W24, DAS28(CRP) <2.6 and ≤3.2 response rates were higher for both doses of FIL vs PBO (
P
<0.05) in all regions, with the exception of Other, where PBO was higher than FIL100 for DAS28(CRP) <2.6 (
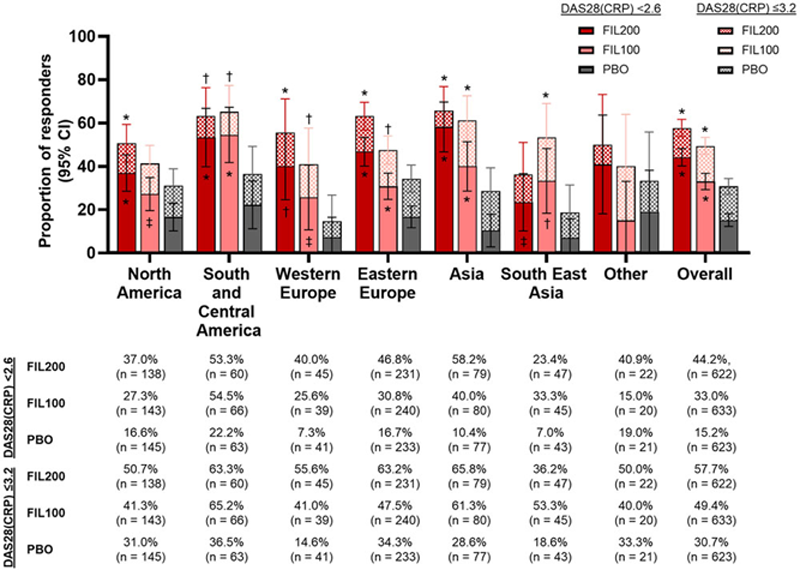
Conclusion: In exploratory analyses, ACR20, DAS28(CRP) <2.6 and ≤3.2 response rates and HAQ-DI scores varied between regions; however, no stable trend was shown in any particular region. Small pt numbers in some subgroups may confound statistical analysis.
REFERENCES:
[1]Genovese et al. JAMA . 2019;322:315–25.
[2]Westhovens et al. Ann Rheum Dis . 2021; online first.
[3]Combe et al. Ann Rheum Dis . 2021; online first.
Disclosure of Interests: Maya H Buch Speakers bureau: AbbVie; Eli Lilly and Company; Gilead Sciences, Inc.; Merck-Serono; Pfizer; Roche; Sandoz; Sanofi; and UCB, Consultant of: AbbVie; Eli Lilly and Company; Gilead Sciences, Inc.; Merck-Serono; Pfizer; Roche; Sandoz; Sanofi; and UCB., Grant/research support from: AbbVie; Eli Lilly and Company; Gilead Sciences, Inc.; Merck-Serono; Pfizer; Roche; Sandoz; Sanofi; and UCB., Tsukasa Matsubara Speakers bureau: Pfizer Japan, Nichi-Iko, Astellas, Meiji Seika, Bristol-Myers Squibb, AbbVie GK, Janssen, Chugai, Eisai, AYUMI, Bernard Combe Speakers bureau: BMS; Eli Lilly & Co.; Gilead Sciences, Inc.; MSD; Pfizer; Roche-Chugai; and UCB, Consultant of: AbbVie; Eli Lilly & Co.; Gilead Sciences, Inc.; Janssen; Pfizer; Roche-Chugai; and Sanofi, Grant/research support from: Novartis, Pfizer, and Roche-Chugai, Alena Pechonkina Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., YingMeei Tan Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Zhaoyu Yin Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Jaehyung Hong Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Robin Besuyen Shareholder of: Galapagos, BV, Employee of: Galapagos, BV, Antonio Gomez-Centeno Speakers bureau: AbbVie, Bristol-Myers Squibb, Eli Lilly & Co., Gebro, Janssen, Menarini, Merck Sharp & Dohme, Pfizer, Roche, Rubio, Sanofi, and UCB, Consultant of: AbbVie, Biogen, Bristol-Myers Squibb, Celgene, Eli Lilly & Co., Gebro, Gilead Sciences, Inc., Hospira, Merck Sharp & Dohme, Pfizer, Roche, Rubio, Sandoz, Sanofi, Grant/research support from: Boehringer Ingelheim, Celltrion, Eli Lilly & Co., Galapagos NV, Gilead Sciences, Inc., Novartis, Pfizer, Roche, Sanofi, UCB, YL Biologics