

Background: Systemic lupus erythematosus (SLE) is a chronic disease characterized by periodic flares associated with poor outcomes and subsequent organ damage (1-2). Flare prevention is important for optimal patient management and development of effective therapies.
Objectives: To identify patient-level factors associated with flares among patients with moderate/severe SLE.
Methods: We conducted a post-hoc analysis of 260 patients with active, autoantibody+ SLE enrolled in a phase II randomized clinical trial (Fenebrutinib) (3). The relationship between baseline demographic (age, gender, ethnicity, BMI), region (US/EU, outside US/EU), disease severity (PGA, SLEDAI-2K, BILAG domain involvement), disease duration, serologic markers (C3, C4, ANA, anti-dsDNA Ab, anti-Smith Ab), treatment arm, standard of care (SOC) and flares (BILAG and SFI) over 48 wks was assessed by survival analysis and multiple Cox Proportional Hazard models. We examined concordance between BILAG and SFI flares using Cohen’s Kappa Index.
Results: The overall rate of flare was low (n=37 SFI flare, n=25 BILAG flare). Median time to first flare was 8 wks for SFI flares compared to 12 wks for BILAG flares. There was no difference in flare rate by treatment arm. Cumulative flare hazard increased over time. Concordance between SFI and BILAG flares was 0.14. Multivariable analyses identified a higher flare rate for both SFI and BILAG-defined flares in patients with severe disease at baseline (PGA >1.7, SLEDAI-2K ≥10) and <7 y disease duration.
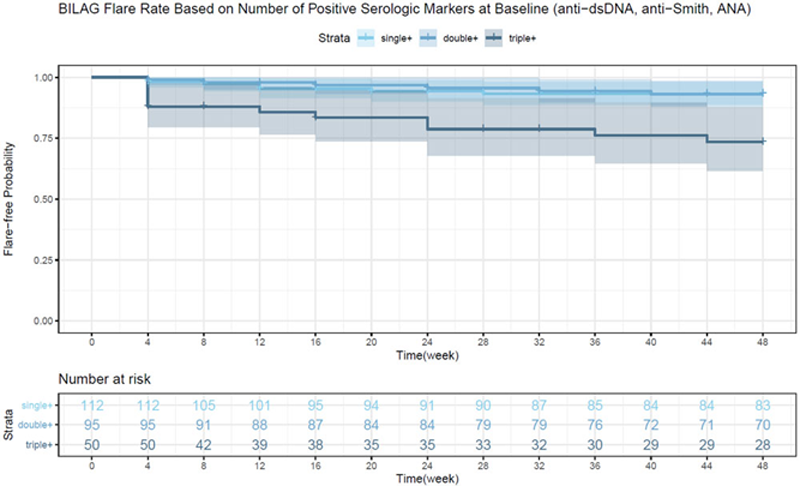
Flares were more common in patients ANA, anti-dsDNA and anti-Smith+ at baseline compared to patients with <3 + markers (p<.001). Furthermore, anti-dsDNA ( p = .03) and/or anti-Smith (p = .001) positivity at baseline were better indicators of higher flare rate compared to ANA ( p = 0.5). Low baseline complement level (C3 and C4) was associated with a higher flare rate (p = .03 and p = .03 respectively).
Patients from non-US/EU regions had a higher flare rate compared to patients from the US/EU, despite receiving more frequent SOC therapy and higher baseline corticosteroid doses (≥10 mg/d). Overall, flare-free probability was comparable at 48 wks regardless of baseline corticosteroid dose but patients receiving <10 mg/d had a median time to flare of 4 vs 24 wks for those receiving ≥10 mg/d (p = .004).
Conclusion: In this study, flares were more common among patients with more severe disease, shorter disease duration, multiple serologic markers, were from outside the US/EU, and received lower steroid doses at baseline.
REFERENCES:
[1]Fernandez D and Kirou KA. Curr Rheumatol Rep 2016 18:14.
[2]Stoll T, et al. Rheum (Oxford) 2004 43(8):1039–44.
[3]Isenberg D, et al. Arth Rheum 2019 71 suppl 10.
| Baseline Factors (% ) | No Flare n=206 | Flare | ||
| BILAG n=25 | SFI n=37 | BILAG and SFI n=8 | ||
| Age (mean (SD)) | 41.8 (12) | 35.2 (9) | 40.4 (10) | 34.9 (8) |
| Female | 199 (97) | 24 (96) | 35 (95) | 7 (88) |
| PGA (mean (SD)) | 1.7 (0.5) | 1.7 (0.4) | 1.9 (0.5) | 1.7 (0.6) |
| BILAG A/B any domain | 197 (96) | 23 (92) | 35 (95) | 7 (88) |
| SLEDAI 2K >=10 | 87 (42) | 18 (72) | 17 (46) | 4 (50) |
| Disease duration (y) (mean (SD)) | 9.4 (7) | 5.3 (4) | 6.6 (6) | 2.9 (3) |
| ANA + | 203 (99) | 24 (96) | 35 (95) | 8 (100) |
| anti-dsDNA + | 102 (50) | 18 (72) | 21 (57) | 5 (63) |
| anti-Smith + | 45 (22) | 13 (52) | 12 (32) | 4 (50) |
| Low C3 | 57 (28) | 12 (48) | 13 (35) | 3 (38) |
| Low C4 | 26 (13) | 7 (28) | 4 (11) | 1 (13) |
| Non US/EU | 157 (76) | 21 (84) | 32 (87) | 8 (100) |
| Corticosteroid | 130 (63) | 14 (56) | 21 (57) | 5 (63) |
| ≥10 mg/d | 80 (39) | 9 (36) | 14 (38) | 4 (50) |
| Immunosuppressant | 74 (36) | 12 (48) | 15 (41) | 3 (38) |
| Antimalarial | 135 (66) | 14 (56) | 21 (57) | 5 (63) |
Notes: included patients 18-75 y; 1+ serologic marker of SLE; SLEDAI-2K >=8, PGA>=1; 1+ oral SOC treatment
SFI = SELENA- SLEDAI Flare Index
Disclosure of Interests: Lisa Lindsay Shareholder of: Employee of Genentech, Inc., Employee of: Employee of Genentech, Inc., Huiyan (Ashley) Mao Shareholder of: Employee of Hoffmann-La Roche Limited, Employee of: Employee of Hoffmann-La Roche Limited, Ji (Emmy) Cheng Shareholder of: Employee of Hoffmann-La Roche Limited, Employee of: Employee of Hoffmann-La Roche Limited, Ching-Yi Chuo Shareholder of: Employee of Genentech, Inc., Employee of: Employee of Genentech, Inc., Nicholas Jones Shareholder of: Employee of Genentech, Inc., Employee of: Employee of Genentech, Inc., Matthew D. Cascino Shareholder of: Employee of Genentech, Inc., Employee of: Employee of Genentech, Inc., Katie Tuckwell Shareholder of: Employee of Genentech, Inc., Employee of: Employee of Genentech, Inc.