

Background: Guselkumab (GUS) is a human monoclonal antibody specific to the p19-subunit of interleukin-23. GUS significantly improved signs and symptoms of PsA through Week24 (Wk24), and improvements were maintained through Wk52 in the Phase 3 DISCOVER-1 1 and DISCOVER-2 2 studies.
Objectives: Assess GUS efficacy through Wk52 in both studies utilizing composite indices.
Methods: Adult patients (pts) enrolled had active PsA despite standard therapies. Pts in DISCOVER-1 had ≥3 swollen and ≥3 tender joints and C-reactive protein (CRP) ≥0.3 mg/dL; in DISCOVER-2, pts had ≥5 swollen and ≥5 tender joints and CRP ≥0.6 mg/dL. 31% of DISCOVER-1 pts received 1-2 prior tumor necrosis factor inhibitors; DISCOVER-2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Wk0, Wk4, then every 8 weeks (Q8W); or placebo (PBO); PBO pts crossed over to GUS 100 mg Q4W at Wk24. Composite endpoints pooled across the two studies were: Disease Activity Index for Psoriatic Arthritis (DAPSA), Psoriatic Arthritis Disease Activity Score (PASDAS), Minimal Disease Activity (MDA), and Very Low Disease Activity (VLDA). GUS vs PBO comparisons through Wk24 employed a Cochran-Mantel-Haenszel test with baseline stratification factors or Fisher’s exact test; no treatment group comparisons were performed beyond Wk24. P-values were not adjusted for multiplicity. From Wk24 -Wk52, pts with missing data were considered nonresponders (>90% of pts completed study treatment through Wk52).
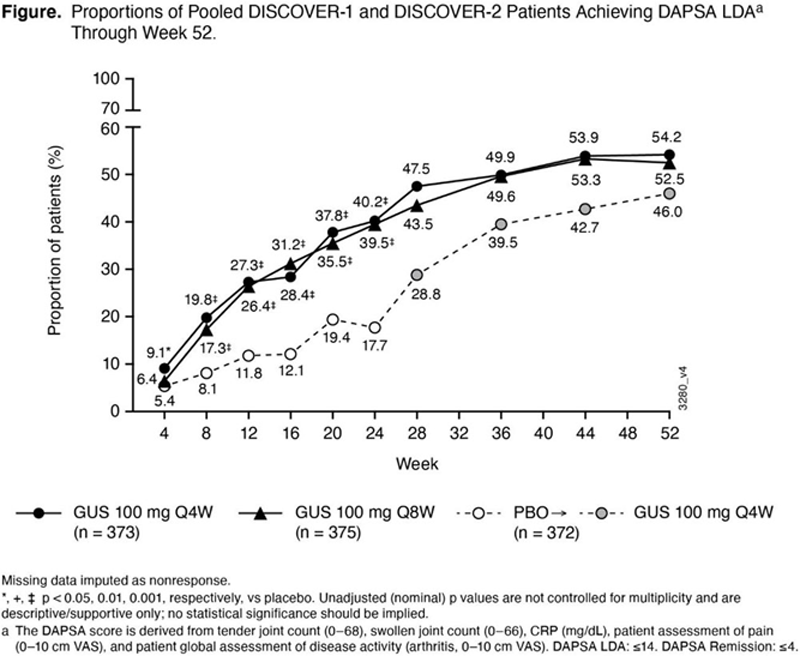
Results: In randomized and treated pts from DISCOVER-1 (N=381) and DISCOVER-2 (N=739), pooled baseline characteristics were generally well-balanced across treatment groups and reflected active disease. Differences in response rates between GUS Q4W or Q8W and PBO were seen as early as Wk8 and increased over time through Wk24. In pts continuing GUS Q4W or Q8W, respectively, post-Wk24 response rates associated with these composite indices continued to increase through Wk52, at which time they were 54.2% and 52.5% for DAPSA LDA, 45.3% and 41.9% for PASDAS LDA, 35.9% and 30.7% for MDA, 18.2% and 17.6% for DAPSA remission, and 13.1% and 14.4% for VLDA, with no discernable difference between the GUS Q4W and Q8W dosing regimens (
Conclusion: GUS 100 mg Q4W and Q8W provided robust and sustained benefits to pts with active PsA across multiple domains, indicating that GUS may provide an alternative treatment option for the diverse manifestations of PsA.
REFERENCES:
[1]Ritchlin CR et al. RMD Open 2021; 1–11. doi: rmdopen-2020-001457
[2]McInnes IB, et al. Arthritis Rheumatol 2020 Oct 11. doi: 10.1002/art.41553.
Pooled response rates for DISCOVER-1 and DISCOVER-2 randomized and treated patients.
| DISCOVER-1&2 | |||
| GUS Q4W | GUS Q8W | PBO -->
|
|
| Randomized and treated patients, n | 373 | 375 | 372 |
| PASDAS 2 LDA | |||
| Wk 24 | 27.9%** | 30.1%** | 8.9% |
| Wk 52 | 45.3% | 41.9% | 36.8% |
| MDA 3 | |||
| Wk 24 | 22.8%** | 24.3%** | 7.8% |
| Wk 52 | 35.9% | 30.7% | 28.2% |
| DAPSA 4 Remission | |||
| Wk 24 | 10.2%** | 8.3%** | 2.2% |
| Wk 52 | 18.2% | 17.6% | 11.0% |
| VLDA 3 | |||
| Wk 24 | 6.4%** | 4.3%* | 1.3% |
| Wk 52 | 13.1% | 14.4% | 8.3% |
Data reported as proportions of patients, %. Unadjusted p values at Wk24 vs PBO: *p<0.05; **p<0.001.
1 Pts randomized to PBO crossed over to GUS Q4W at Wk24.
2 PASDAS is derived from Pt global assessment of arthritis and psoriasis (0-100), Physician global assessment (0-100), swollen joint count (0-66), tender joint count (0-68), CRP (mg/L), Leeds enthesitis index score, tender dactylitis count, and the 36-item Short-Form Health Survey Physical Component Summary score. PASDAS LDA ≤3.2.
3 MDA is 5/7 criteria met; VLDA is 7/7 criteria met: tender joint count ≤1, swollen joint count ≤1, Psoriasis Activity and Severity Index ≤1, Pt assessment of pain ≤15 (0-100), Pt global assessment of disease activity ≤20 (0-100), Health Assessment Questionnaire-Disability Index score ≤0.5, Tender entheseal points ≤1.
4
DAPSA Remission: score ≤4 (definition in
Disclosure of Interests: Laura C Coates Consultant of: AbbVie, Amgen, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Domain, Eli Lilly, Gilead, Janssen, Medac, Novartis, Pfizer and UCB, Grant/research support from: AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Novartis, Pfizer, Christopher T. Ritchlin Consultant of: Amgen, AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Amgen, and UCB, Laure Gossec Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Biogen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis, and UCB., Grant/research support from: Amgen, Eli Lilly, Galapagos, Janssen, Pfizer, Sandoz, and Sanofi, Philip Helliwell Consultant of: Galapagos, Janssen, and Novartis, Grant/research support from: AbbVie, Janssen, Pfizer, Proton Rahman Speakers bureau: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB, Grant/research support from: Janssen and Novartis, Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Chetan Karyekar Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, Wim Noel Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC, Yusang Jiang Employee of: Cytel, Inc. providing statistical support (funded by Janssen), Shihong Sheng Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Yanli Wang Employee of: IQVIA providing statistical support (funded by Janssen), Philip J Mease Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, SUN, and UCB.