

Background: DISCOVER-2 was a Phase 3 trial of the first-in-class anti-IL-23-specific mAb guselkumab (GUS) in patients (pts) with psoriatic arthritis (PsA). PsA impacts patients’ productivity at work and in daily activity.1
Objectives: To evaluate the effect of GUS on work productivity and daily activity in DISCOVER-2 through 1 year using the Work Productivity and Activity Impairment Questionnaire: PsA (WPAI- PsA).
Methods: Bio-naïve adults with active PsA despite nonbiologic DMARDs &/or NSAIDs received subcutaneous GUS 100 mg every 4 weeks (Q4W); GUS 100 mg W0, W4, then Q8W; or placebo (PBO). At W24, PBO pts crossed over to GUS 100 mg Q4W. WPAI-PsA assesses PsA-related work time missed (absenteeism), impairment while working (presenteeism), impaired overall work productivity (absenteeism + presenteeism), and daily activity during the previous week. A shift analysis evaluated proportions of pts employed vs unemployed (regardless of desire to work) over time. Among pts working at baseline, least-squares (LS) mean changes from baseline in WPAI-PsA domains were determined using a mixed-effects model for repeated measures analysis, whereby mean changes in WPAI-PsA domains were calculated for each multiple imputation (MI) dataset using an analysis of covariance (ANCOVA); the reported LSmean is the average of all MI datasets. Also, among pts employed at baseline, indirect savings from improved overall work productivity were estimated using 2020 EU mean yearly wage estimate (all occupations).2
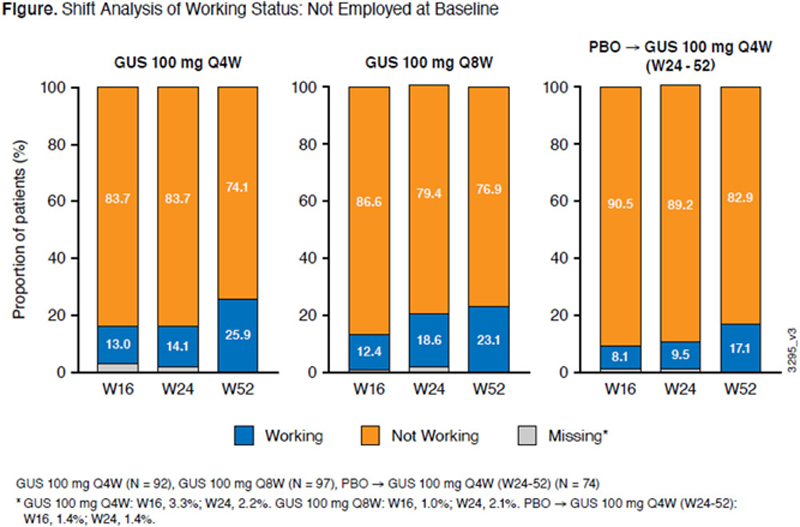
Results: In pts working at baseline, significant improvement in work productivity and non-work activity vs PBO was observed at W24. Productivity gains seen with GUS at W24 continued to improve through 1 year (
Conclusion: Improvement in work productivity and non-work activity was greater with GUS vs PBO among pts with active PsA through W52. Improvements demonstrated may result in reduction in PsA costs associated with work productivity.
REFERENCES:
[1]Tillett W et al. Rheumatol (Oxford). 2012;51:275–83.
[2]OECD (2020). Average wages (indicator).
Model-based estimates of LSmean changea (95% CI) from baseline in WPAI-PsA domains among pts working at baseline and with an observed change through W24 (N=474) and W52 (N=475)
| Change from baseline | GUS 100mg Q4W | GUS 100mg Q8W |
PBO
| PBO → GUS 100 mg Q4W (W24-52 ) | ||
| Visit | W24 | W52 | W24 | W52 | W24 | W52 |
| Absenteeism, N | 145 | 145 | 147 | 147 | 162 | 163 |
| LSmean | -3.4 (-6.5,-0.3) | -4.1 (-6.8,-1.5) | -3.0 (-6.0,0.1) | -4.0 (-6.6,-1.3) | -3.0 (-6.0, 0.04) | -3.0 (-5.5,-0.4) |
| Diff vs. PBO | -0.4 (-4.6,3.8) | -0.01 (-4.2, 4.2) | ||||
| Presenteeism, N | 145 | 145 | 147 | 147 | 162 | 163 |
| LSmean | -20.1 (-23.7,-16.6) | -22.4 (-26.3,-18.6) | -19.6 (-23.2,-16.1) | -25.7 (-29.5,-21.8) | -10.5 (-13.9,-7.0) | -18.5 (-22.2,-14.7) |
| Diff vs PBO | -9.7* (-14.4,-5.0) | -9.2* (-13.9,-4.5) | ||||
| Work productivity, N | 145 | 145 | 147 | 147 | 162 | 163 |
| LSmean | -20.1 (-24.1,-16.1) | -22.6 (-26.8,-18.3) | -19.2 (-23.1,-15.2) | -25.9 (-30.0,-21.7) | -10.6 (-14.4,-6.8) | -17.6 (-21.7,-13.6) |
| Diff vs PBO | -9.5* (-14.8,-4.2) | -8.6* (-13.9,-3.3) | ||||
| Non-work Activity, N | 242 | 242 | 246 | 246 | 245 | 245 |
| LSmean | -20.5 (-23.3,-17.7) | -25.7 (-28.6,-22.7) | -21.2 (-23.9,-18.4) | -25.4 (-28.4,-22.5) | -9.9 (-12.6,-7.1) | -22.3 (-25.3,-19.4) |
| Diff vs PBO | -10.6* (-14.4,-6.8) | -11.3* (-15.1,-7.5) | ||||
CI=Confidence interval
a. LSmean for each MI dataset is calculated based on an ANCOVA model for the change from baseline at W24/W52. The combined LSmean, which is the average of the LSmean, taken over all the MI datasets, is presented.
*p<0.05
Disclosure of Interests: Jeffrey Curtis Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB, Iain McInnes Consultant of: AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, and UCB, Steve Peterson Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, Prasheen Agarwal Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Feifei Yang Shareholder of: Johnson & Johnson, Employee of: Janssen Global Services, LLC, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Elizabeth C Hsia Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Chenglong Han Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, William Tillett Speakers bureau: AbbVie, Amgen, Celgene, Lilly, Janssen, Novartis, Pfizer Inc, and UCB, Consultant of: AbbVie, Amgen, Celgene, Lilly, Janssen, Novartis, MSD, Pfizer Inc, and UCB, Grant/research support from: AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB, Philip J Mease Speakers bureau: Boehringer Ingelheim and GlaxoSmithKline, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, SUN, and UCB, Proton Rahman Speakers bureau: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB, Grant/research support from: Janssen and Novartis.