

Background: PATERA is an ongoing phase 3 international double-blind, placebo-controlled clinical study of netakimab (NTK) in psoriatic arthritis (PsA) (NCT03598751). Netakimab (NTK) is an anti-interleukin-17A monoclonal antibody approved for psoriasis, ankylosing spondylitis, PsA in Russia and Belarus.
Objectives: A subanalysis was performed to define the impact of NTK on PsA activity depending on the presence of axial disease: a subset of patients with inflammatory back pain (IBP) according to self-reported ASAS IBP criteria, 2009 (IBP(+) was compared to those without IBP (IBP(-)).
Methods: 194 eligible adult patients with PsA fulfilling the CASPAR criteria, with inadequate response to csDMARD or one TNFi, were randomly assigned to receive NTK 120mg or placebo at weeks 0,1,2,4,6,8,10,14,18,22. Patients with missing values for categorical variables were considered as non-responders in the analysis. For quantitative variables, missing values were replaced using the multiple imputation method.
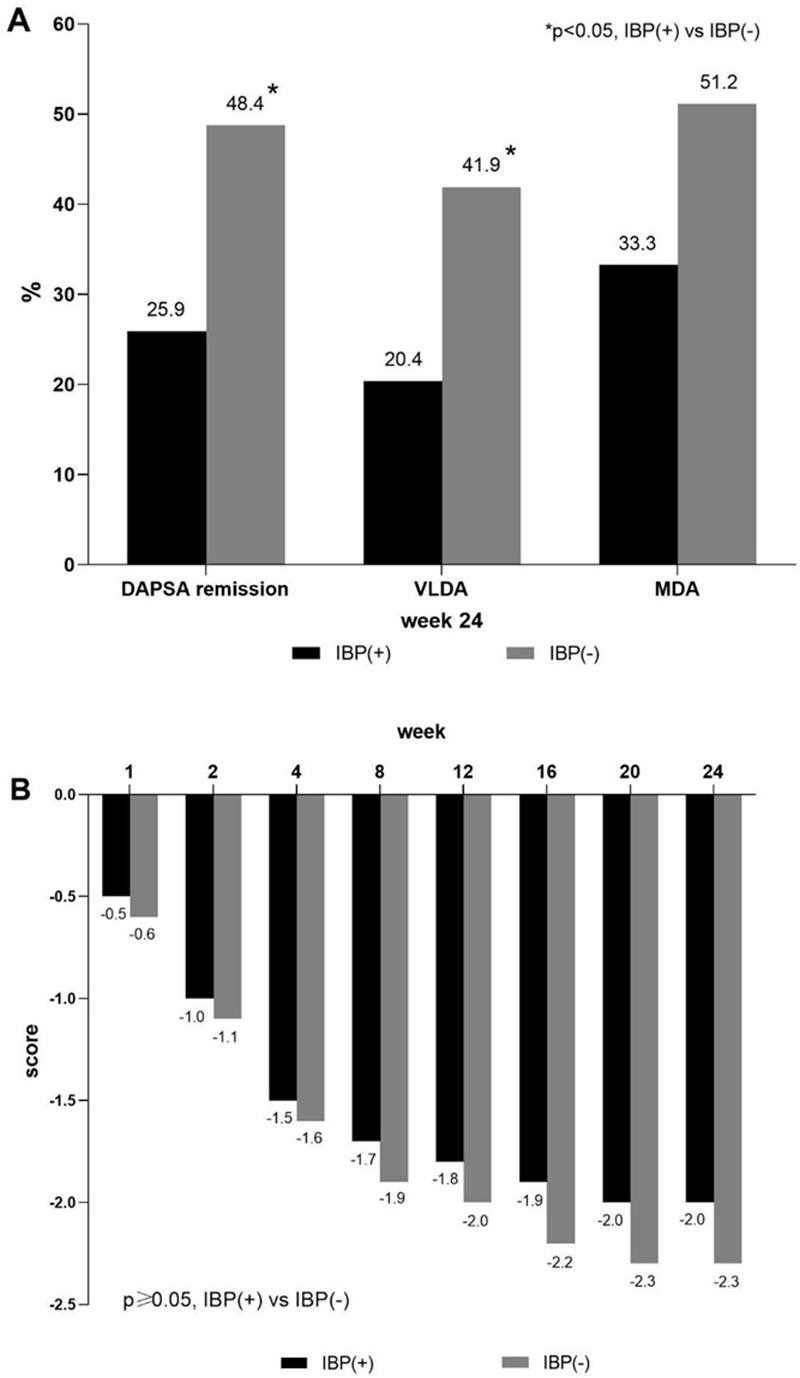
Results: 97 PsA patients (N=54 IBP(+), N=43 IBP(-)) received NTK. Both subpopulations were comparable in gender, age and PsA activity at baseline. The treatment led to a pronounced decline in PsA activity in both subpopulations, significant differences between arms were observed only in DAPSA remission and very low disease activity (VLDA) at week 24 (
Conclusion: NTK significantly improved PsA activity regardless of the presence of IBP.
PsA activity after 24-week treatment with NTK. (A) Percentage of patients with DAPSA remission (0-4), very low disease activity (VLDA), minimal disease activity (MDA) at week 24; (B) change from baseline in DAS28-CRP
Acknowledgements: This study was sponsored by JSC BIOCAD.
Disclosure of Interests: Tatiana Korotaeva Speakers bureau: Abbvie, Biocad, Eli Lilly, Johnson & Johnson, Janssen, Novartis, Pfizer, UCB, Inna Gaydukova Speakers bureau: Abbvie, Biocad, Eli Lilly, MSD, Novartis, Pfizer, Sandoz, V Mazurov: None declared, Aleksey Samtsov: None declared, Vladislav Khayrutdinov: None declared, Andrey Bakulev: None declared, Alena Kundzer: None declared, Nikolaj Soroka: None declared, Anna Eremeeva Employee of: Biocad.